Remodeling and shuttling. Mechanisms for the synergistic effects between different acceptor particles in the mobilization of cellular cholesterol
- PMID: 9081695
- PMCID: PMC5021317
- DOI: 10.1161/01.atv.17.2.383
Remodeling and shuttling. Mechanisms for the synergistic effects between different acceptor particles in the mobilization of cellular cholesterol
Abstract
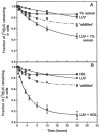
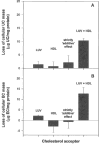
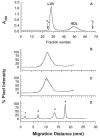
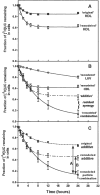
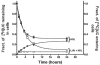
In normal physiology, cells are exposed to cholesterol acceptors of different sizes simultaneously. The current study examined the possible interactions between two different classes of acceptors, one large (large unilamellar phospholipid vesicles, LUVs) and one small (HDL or other small acceptors), added separately or in combination to Fu5AH rat hepatoma cells. During a 24-hour incubation, LUVs of palmitoyl-oleoyl phosphatidylcholine at 1 mg phospholipid (PL) per milliliter extracted approximately 20% of cellular unesterified cholesterol (UC) label and mass in a slow, continuous fashion (half-time [t1/2] for UC efflux was approximately 50 hours) and human HDL3 at 25 micrograms PL per milliliter extracted approximately 15% cellular UC label with no change in cellular cholesterol mass (t1/2 of approximately 8 hours). In contrast, the combination of LUVs and HDL3 extracted over 90% of UC label (t1/2 of approximately 4 hours) and approximately 50% of the UC mass, indicating synergy. To explain this synergy, specific particle interactions were examined, namely, remodeling, in which the two acceptors alter each other's composition and thus the ability to mobilize cellular cholesterol, and shuttling, in which the small acceptor ferries cholesterol from cells to the large acceptor. To examine remodeling, LUVs and HDL were coincubated and reisolated before application to cells. This HDL became UC depleted, PL enriched, and lost a small amount of apolipoprotein A-I. Compared with equivalent numbers of control HDL particles; remodeled HDL caused faster efflux (t1/2 approximately 4 hours) and exhibited a greater capacity to sequester cellular cholesterol over 24 hours (approximately 38% versus approximately 15% for control HDL), consistent with their enrichment in PL. Remodeled LUVs still extracted approximately 20% of cellular UC. Thus, remodeling accounted for some but not all of the synergy between LUVs and HDL. To examine shuttling, several approaches were used. First, reisolation of particles after an 8-hour exposure to cells revealed that HDL contained very little of the cellular UC label. The label was found almost entirely with the LUVs, suggesting that LUVs continuously stripped the HDL of cellular UC. Second, bidirectional flux studies demonstrated that LUVs blocked the influx of HDL UC label into cells, while the rate of efflux of cellular UC was maintained. These kinetic effects explained the massive net loss of cellular UC to LUVs with HDL. Third, cyclodextrin, an artificial small acceptor that does not acquire PL and hence does not become remodeled, exhibited substantial synergy with LUVs, supporting shuttling. Thus, the presence of large and small acceptors together can overcome intrinsic deficiencies in each. Small acceptors are efficient at extracting cellular cholesterol because they approach cell surfaces easily but have a low capacity, whereas large acceptors are inefficient but have a high capacity. When present simultaneously, where the small acceptor can transfer cholesterol quickly to the large acceptor, high efficiency and high capacity are achieved. The processes responsible for this synergy, namely, remodeling and shuttling, may be general phenomena allowing cooperation both during normal physiology and after therapeutic administration of acceptors to accelerate tissue cholesterol efflux in vivo.
Figures
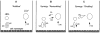
Similar articles
-
Effects of acceptor particle size on the efflux of cellular free cholesterol.J Biol Chem. 1995 Jul 21;270(29):17106-113. doi: 10.1074/jbc.270.29.17106. J Biol Chem. 1995. PMID: 7615505
-
The effect of high density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol.J Biol Chem. 1995 Mar 17;270(11):5882-90. doi: 10.1074/jbc.270.11.5882. J Biol Chem. 1995. PMID: 7890719
-
The bidirectional flux of cholesterol between cells and lipoproteins. Effects of phospholipid depletion of high density lipoprotein.J Biol Chem. 1986 May 5;261(13):5766-76. J Biol Chem. 1986. PMID: 3700371
-
New insights into the regulation of HDL metabolism and reverse cholesterol transport.Circ Res. 2005 Jun 24;96(12):1221-32. doi: 10.1161/01.RES.0000170946.56981.5c. Circ Res. 2005. PMID: 15976321 Review.
-
Lipoproteins and cellular cholesterol homeostasis.Subcell Biochem. 1997;28:235-76. doi: 10.1007/978-1-4615-5901-6_9. Subcell Biochem. 1997. PMID: 9090297 Review.
Cited by
-
Interaction between VLDL and phosphatidylcholine liposomes generates new γ-LpE-like particles.Lipids. 2014 Feb;49(2):143-53. doi: 10.1007/s11745-013-3861-8. Lipids. 2014. PMID: 24234844 Free PMC article.
-
Molecular mechanisms of cellular cholesterol efflux.J Biol Chem. 2014 Aug 29;289(35):24020-9. doi: 10.1074/jbc.R114.583658. Epub 2014 Jul 29. J Biol Chem. 2014. PMID: 25074931 Free PMC article. Review.
-
Syndecans reside in sphingomyelin-enriched low-density fractions of the plasma membrane isolated from a parathyroid cell line.PLoS One. 2012;7(3):e32351. doi: 10.1371/journal.pone.0032351. Epub 2012 Mar 1. PLoS One. 2012. PMID: 22396758 Free PMC article.
-
Identification of haptoglobin and apolipoprotein A-I as biomarkers for high altitude pulmonary edema.Funct Integr Genomics. 2011 Sep;11(3):407-17. doi: 10.1007/s10142-011-0234-3. Epub 2011 Jul 14. Funct Integr Genomics. 2011. PMID: 21755356
-
Different Pathways of Cellular Cholesterol Efflux.Cell Biochem Biophys. 2022 Sep;80(3):471-481. doi: 10.1007/s12013-022-01081-5. Epub 2022 Jun 23. Cell Biochem Biophys. 2022. PMID: 35737216 Review.
References
-
- Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. - PubMed
-
- Miller NE. HDL metabolism and its role in lipid transport. Eur Hearl J. 1990;11(suppl H):1–3. - PubMed
-
- Pieters MN, Schonten D, Van Berkel TJ. In vitro and in vivo evidence for the role of HDL in reverse cholesterol transport. Biochim Biophys Acta. 1994;1225:125–134. - PubMed
-
- Castro GR, Fielding CJ. Early incorporation of cell-derived cholesterol into pre-beta–migrating high-density lipoprotein. Biochemistiy. 1988;27:25–29. - PubMed
-
- Francone OL, Fielding CJ. Initial steps in reverse cholesterol transport: the role of short-lived cholesterol acceptors. Eur Heart J. 1990;11(suppl E):218–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

