Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts: implications for the generation of motile force
- PMID: 9087444
- PMCID: PMC2132518
- DOI: 10.1083/jcb.136.6.1287
Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts: implications for the generation of motile force
Abstract
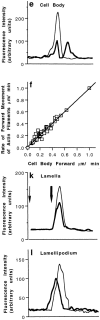
We have determined the structural organization and dynamic behavior of actin filaments in entire primary locomoting heart fibroblasts by S1 decoration, serial section EM, and photoactivation of fluorescence. As expected, actin filaments in the lamellipodium of these cells have uniform polarity with barbed ends facing forward. In the lamella, cell body, and tail there are two observable types of actin filament organization. A less abundant type is located on the inner surface of the plasma membrane and is composed of short, overlapping actin bundles (0.25-2.5 microm) that repeatedly alternate in polarity from uniform barbed ends forward to uniform pointed ends forward. This type of organization is similar to the organization we show for actin filament bundles (stress fibers) in nonlocomoting cells (PtK2 cells) and to the known organization of muscle sarcomeres. The more abundant type of actin filament organization in locomoting heart fibroblasts is mostly ventrally located and is composed of long, overlapping bundles (average 13 microm, but can reach up to about 30 microm) which span the length of the cell. This more abundant type has a novel graded polarity organization. In each actin bundle, polarity gradually changes along the length of the bundle. Actual actin filament polarity at any given point in the bundle is determined by position in the cell; the closer to the front of the cell the more barbed ends of actin filaments face forward. By photoactivation marking in locomoting heart fibroblasts, as expected in the lamellipodium, actin filaments flow rearward with respect to substrate. In the lamella, all marked and observed actin filaments remain stationary with respect to substrate as the fibroblast locomotes. In the cell body of locomoting fibroblasts there are two dynamic populations of actin filaments: one remains stationary and the other moves forward with respect to substrate at the rate of the cell body. This is the first time that the structural organization and dynamics of actin filaments have been determined in an entire locomoting cell. The organization, dynamics, and relative abundance of graded polarity actin filament bundles have important implications for the generation of motile force during primary heart fibroblast locomotion.
Figures










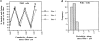
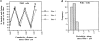
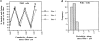




References
-
- Abercrombie M. The crawling movements of metazoan cells. Proc R Soc Lond Ser B. 1980;207:129–147.
-
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. II “Ruffling”. Exp Cell Res. 1970;60:437–444. - PubMed
-
- Boyles J, Anderson L, Hutcherson P. A new fixative for the preservation of actin filaments: fixation of pure actin filament pellets. J Histochem Cytochem. 1985;33:1116–1128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

