Xenopus actin depolymerizing factor/cofilin (XAC) is responsible for the turnover of actin filaments in Listeria monocytogenes tails
- PMID: 9087446
- PMCID: PMC2132508
- DOI: 10.1083/jcb.136.6.1323
Xenopus actin depolymerizing factor/cofilin (XAC) is responsible for the turnover of actin filaments in Listeria monocytogenes tails
Abstract
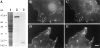
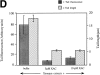
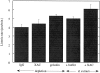
In contrast to the slow rate of depolymerization of pure actin in vitro, populations of actin filaments in vivo turn over rapidly. Therefore, the rate of actin depolymerization must be accelerated by one or more factors in the cell. Since the actin dynamics in Listeria monocytogenes tails bear many similarities to those in the lamellipodia of moving cells, we have used Listeria as a model system to isolate factors required for regulating the rapid actin filament turnover involved in cell migration. Using a cell-free Xenopus egg extract system to reproduce the Listeria movement seen in a cell, we depleted candidate depolymerizing proteins and analyzed the effect that their removal had on the morphology of Listeria tails. Immunodepletion of Xenopus actin depolymerizing factor (ADF)/cofilin (XAC) from Xenopus egg extracts resulted in Listeria tails that were approximately five times longer than the tails from undepleted extracts. Depletion of XAC did not affect the tail assembly rate, suggesting that the increased tail length was caused by an inhibition of actin filament depolymerization. Immunodepletion of Xenopus gelsolin had no effect on either tail length or assembly rate. Addition of recombinant wild-type XAC or chick ADF protein to XAC-depleted extracts restored the tail length to that of control extracts, while addition of mutant ADF S3E that mimics the phosphorylated, inactive form of ADF did not reduce the tail length. Addition of excess wild-type XAC to Xenopus egg extracts reduced the length of Listeria tails to a limited extent. These observations show that XAC but not gelsolin is essential for depolymerizing actin filaments that rapidly turn over in Xenopus extracts. We also show that while the depolymerizing activities of XAC and Xenopus extract are effective at depolymerizing normal filaments containing ADP, they are unable to completely depolymerize actin filaments containing AMPPNP, a slowly hydrolyzible ATP analog. This observation suggests that the substrate for XAC is the ADP-bound subunit of actin and that the lifetime of a filament is controlled by its nucleotide content.
Figures
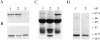






Comment in
-
Accelerating on a treadmill: ADF/cofilin promotes rapid actin filament turnover in the dynamic cytoskeleton.J Cell Biol. 1997 Mar 24;136(6):1165-8. doi: 10.1083/jcb.136.6.1165. J Cell Biol. 1997. PMID: 9087434 Free PMC article. Review. No abstract available.
References
-
- Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J Biol Chem. 1995;270:17582–17587. - PubMed
-
- André E, Lottspeich F, Schleicher M, Noegel A. Severin, gelsolin, and villin share a homologous sequence in regions presumed to contain F-actin severing domains. J Biol Chem. 1988;263:722–727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

