Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal organogenesis in vitro
- PMID: 9087449
- PMCID: PMC2132511
- DOI: 10.1083/jcb.136.6.1363
Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal organogenesis in vitro
Abstract
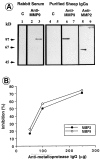
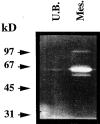
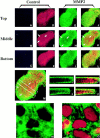
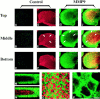
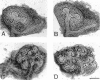
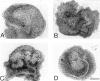
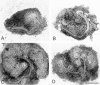
We analyzed matrix metalloproteinase (MMP) production by 11-d embryonic mouse kidneys and the effects of these enzymes on subsequent renal organogenesis. In vivo, immunolocalization of metalloproteinases by laser scanning confocal microscopy and zymograms of kidney lysates showed that the mesenchyme of embryonic kidneys synthesized both MMP9 and MMP2 enzymes. In vitro, embryonic kidneys also secreted both enzymes when cultured in a medium devoid of hormone, growth factor, and serum for 24 h during which T-shaped branching of the ureter bud appeared. We then evaluated the role of MMP2 and MMP9 in kidney morphogenesis by adding anti-MMP2 or anti-MMP9 IgGs to the culture medium of 11-d kidneys for 24 or 72 h. Although it inhibited activity of the mouse enzyme, anti-MMP2 IgGs had no effect on kidney morphogenesis. In contrast, anti-MMP9 IgGs with enzyme-blocking activity impaired renal morphogenesis, in a concentration-dependent manner, by inhibiting T-shaped branching and further divisions of the ureter bud. This effect was irreversible, still observed after inductive events and reproduced by exogenous tissue inhibitor of metalloproteinase 1 (TIMP1), the natural inhibitor of MMP9. These data provide the first demonstration of MMP9 and MMP2 production in vivo by 11-d embryonic kidneys and further show that MMP9 is required in vitro for branching morphogenesis of the ureter bud.
Figures

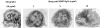
Similar articles
-
Expression of the type IV collagenase system during mouse kidney development and tubule segmentation.J Am Soc Nephrol. 2001 Nov;12(11):2358-2369. doi: 10.1681/ASN.V12112358. J Am Soc Nephrol. 2001. PMID: 11675412
-
Matrix metalloproteinase 2 (MMP2) and MMP9 are produced by kidney collecting duct principal cells but are differentially regulated by SV40 large-T, arginine vasopressin, and epidermal growth factor.J Biol Chem. 1999 Jan 15;274(3):1614-20. doi: 10.1074/jbc.274.3.1614. J Biol Chem. 1999. PMID: 9880540
-
Matrix metalloproteinases and their inhibitors regulate in vitro ureteric bud branching morphogenesis.Am J Physiol Renal Physiol. 2000 Nov;279(5):F891-900. doi: 10.1152/ajprenal.2000.279.5.F891. Am J Physiol Renal Physiol. 2000. PMID: 11053050
-
Cloning of murine membrane-type-1-matrix metalloproteinase (MT-1-MMP) and its metanephric developmental regulation with respect to MMP-2 and its inhibitor.Kidney Int. 1998 Jul;54(1):131-42. doi: 10.1046/j.1523-1755.1998.00XXX.x. Kidney Int. 1998. PMID: 9648071
-
Cyclic AMP-regulated synthesis of the tissue inhibitors of metalloproteinases suppresses the invasive potential of the human fibrosarcoma cell line HT1080.Cancer Res. 1995 Jul 1;55(13):2927-35. Cancer Res. 1995. PMID: 7796421
Cited by
-
MMP-2 mediates local degradation and remodeling of collagen by annulus fibrosus cells of the intervertebral disc.Arthritis Res Ther. 2013 Apr 27;15(2):R57. doi: 10.1186/ar4224. Arthritis Res Ther. 2013. PMID: 23621950 Free PMC article.
-
MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix.J Cell Biol. 2004 Nov 22;167(4):757-67. doi: 10.1083/jcb.200405001. Epub 2004 Nov 15. J Cell Biol. 2004. PMID: 15545316 Free PMC article.
-
Macrophages and CSF-1: implications for development and beyond.Organogenesis. 2013 Oct 1;9(4):249-60. doi: 10.4161/org.25676. Epub 2013 Jul 29. Organogenesis. 2013. PMID: 23974218 Free PMC article. Review.
-
Expression of matrix metalloproteinase-2 and matrix metalloproteinase-9 in recurrent chronic rhinosinusitis with nasal polyposis.Kaohsiung J Med Sci. 2013 Jan;29(1):26-31. doi: 10.1016/j.kjms.2012.08.004. Epub 2012 Oct 7. Kaohsiung J Med Sci. 2013. PMID: 23257253 Free PMC article.
-
Matrix Metalloproteinase 20 Co-expression With Dentin Sialophosphoprotein in Human and Monkey Kidneys.J Histochem Cytochem. 2016 Oct;64(10):623-36. doi: 10.1369/0022155416665098. J Histochem Cytochem. 2016. PMID: 27666430 Free PMC article.
References
-
- Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, Werb Z. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development (Camb) 1996;122:1723–1736. - PubMed
-
- Apte SS, Hayashi K, Seldin MF, Matei M-G, Hayashi M, Olsen BR. Gene encoding a novel murine tissue inhibitor of metalloproteinases (TIMP), TIMP3, is expressed in developing mouse epithelia, cartilage, and muscle, and is located on mouse chromosome 10. Dev Dyn. 1994;200:177–197. - PubMed
-
- Arribas J, Coodly L, Vollmer P, Kishimoto TK, Rose-John S, Massagué J. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem. 1996;271:11376–11382. - PubMed
-
- Bard, J.B.L. 1990. The Cellular and Molecular Processes of Developmental Anatomy. Cambridge University Press, Cambridge, UK. 291 pp. - PubMed
-
- Brenner CA, Adler RR, Rappolee DA, Pederson RA, Werb Z. Genes for extracellular matrix-degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes & Dev. 1989;3:848–859. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

