Locating the anion-selectivity filter of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel
- PMID: 9089437
- PMCID: PMC2217075
- DOI: 10.1085/jgp.109.3.289
Locating the anion-selectivity filter of the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel
Abstract
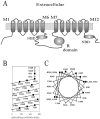
The cystic fibrosis transmembrane conductance regulator forms an anion-selective channel; the site and mechanism of charge selectivity is unknown. We previously reported that cysteines substituted, one at a time, for Ile331, Leu333, Arg334, Lys335, Phe337, Ser341, Ile344, Arg347, Thr351, Arg352, and Gln353, in and flanking the sixth membrane-spanning segment (M6), reacted with charged, sulfhydryl-specific, methanethiosulfonate (MTS) reagents. We inferred that these residues are on the water-accessible surface of the protein and may line the ion channel. We have now measured the voltage-dependence of the reaction rates of the MTS reagents with the accessible, engineering cysteines. By comparing the reaction rates of negatively and positively charged MTS reagents with these cysteines, we measured the extent of anion selectivity from the extracellular end of the channel to eight of the accessible residues. We show that the major site determining anion vs. cation selectivity is near the cytoplasmic end of the channel; it favors anions by approximately 25-fold and may involve the residues Arg347 and Arg 352. From the voltage dependence of the reaction rates, we calculated the electrical distance to the accessible residues. For the residues from Leu333 to Ser341 the electrical distance is not significantly different than zero; it is significantly different than zero for the residues Thr351 to Gln353. The maximum electrical distance measured was 0.6 suggesting that the channel extends more cytoplasmically and may include residues flanking the cytoplasmic end of the M6 segment. Furthermore, the electrical distance calculations indicate that R352C is closer to the extracellular end of the channel than either of the adjacent residues. We speculate that the cytoplasmic end of the M6 segment may loop back into the channel narrowing the lumen and thereby forming both the major resistance to current flow and the anion-selectivity filter.
Figures





References
-
- Akabas MH, Kaufmann C, Archdeacon P, Karlin A. Identification of acetylcholine receptor channel-lining residues in the entire M2 segment of the α subunit. Neuron. 1994a;13:919–927. - PubMed
-
- Akabas MH, Kaufmann C, Cook TA, Archdeacon P. Amino acid residues lining the chloride channel of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1994b;269:14865–14868. - PubMed
-
- Akabas MH, Stauffer DA, Xu M, Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science (Wash DC) 1992;258:307–310. - PubMed
-
- Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Nucleotide triphosphates are required to open the CFTR chloride channel. Cell. 1991a;67:775–784. - PubMed
-
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science (Wash DC) 1991b;253:202–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

