Light adaptation in Pecten hyperpolarizing photoreceptors. Insensitivity to calcium manipulations
- PMID: 9089443
- PMCID: PMC2217071
- DOI: 10.1085/jgp.109.3.371
Light adaptation in Pecten hyperpolarizing photoreceptors. Insensitivity to calcium manipulations
Abstract
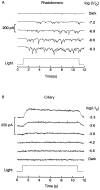
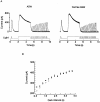
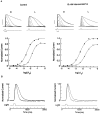
The ability of scallop hyperpolarizing photoreceptors to respond without attenuation to repetitive flashes, together with their low light sensitivity, lack of resolvable quantum bumps and fast photoresponse kinetics, had prompted the suggestion that these cells may be constitutively in a state akin to light adaptation. We here demonstrate that their photocurrent displays all manifestations of sensory adaptation: (a) The response amplitude to a test flash is decreased in a graded way by background or conditioning lights. This attenuation of the response develops with a time constant of 200-800 ms, inversely related to background intensity. (b) Adapting stimuli shift the stimulus-response curve and reduce the size of the saturating photocurrent. (c) The fall kinetics of the photoresponse are accelerated by light adaptation, and the roll-of of the modulation transfer function is displaced to higher frequencies. This light-induced desensitization exhibits a rapid recovery, on the order of a few seconds. Based on the notion that Ca mediates light adaptation in other cells, we examined the consequences of manipulating this ion. Removal of external Ca reversibly increased the photocurrent amplitude, without affecting light sensitivity, photoresponse kinetics, or susceptibility to background adaptation; the effect, therefore, concerns ion permeation, rather than the regulation of the visual response. Intracellular dialysis with 10 mM BAPTA did not reduce the peak-to-plateau decay of the photocurrent elicited by prolonged light steps, not the background-induced compression of the response amplitude range and the acceleration of its kinetics. Conversely, high levels of buffered free [Ca]i (10 microM) only marginally shifted the sensitivity curve (delta sigma = 0.3 log) and spared all manifestations of light adaptation. These results indicate that hyperpolarizing invertebrate photoreceptors adapt to light, but the underlying mechanisms must utilize pathways that are largely independent of changes in cytosolic Ca. The results are discussed in terms of aspects of commonalty to other ciliary sensory receptor cells.
Figures






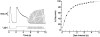



Similar articles
-
Divalent cation interactions with light-dependent K channels. Kinetics of voltage-dependent block and requirement for an open pore.J Gen Physiol. 1999 Nov;114(5):653-72. doi: 10.1085/jgp.114.5.653. J Gen Physiol. 1999. PMID: 10532963 Free PMC article.
-
Calcium-independent, cGMP-mediated light adaptation in invertebrate ciliary photoreceptors.J Neurosci. 2005 Feb 23;25(8):2042-9. doi: 10.1523/JNEUROSCI.5129-04.2005. J Neurosci. 2005. PMID: 15728844 Free PMC article.
-
Ion permeation through light-activated channels in rhabdomeric photoreceptors. Role of divalent cations.J Gen Physiol. 1996 Jun;107(6):715-30. doi: 10.1085/jgp.107.6.715. J Gen Physiol. 1996. PMID: 8783072 Free PMC article.
-
The Limulus ventral photoreceptor: light response and the role of calcium in a classic preparation.Prog Neurobiol. 1997 Nov;53(4):451-515. doi: 10.1016/s0301-0082(97)00046-4. Prog Neurobiol. 1997. PMID: 9421832 Review.
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
Cited by
-
Divalent cation interactions with light-dependent K channels. Kinetics of voltage-dependent block and requirement for an open pore.J Gen Physiol. 1999 Nov;114(5):653-72. doi: 10.1085/jgp.114.5.653. J Gen Physiol. 1999. PMID: 10532963 Free PMC article.
-
Light transduction in invertebrate hyperpolarizing photoreceptors: possible involvement of a Go-regulated guanylate cyclase.J Neurosci. 2000 Jul 15;20(14):5254-63. doi: 10.1523/JNEUROSCI.20-14-05254.2000. J Neurosci. 2000. PMID: 10884309 Free PMC article.
-
Calcium-independent, cGMP-mediated light adaptation in invertebrate ciliary photoreceptors.J Neurosci. 2005 Feb 23;25(8):2042-9. doi: 10.1523/JNEUROSCI.5129-04.2005. J Neurosci. 2005. PMID: 15728844 Free PMC article.
-
Molecular and functional identification of a novel photopigment in Pecten ciliary photoreceptors.J Gen Physiol. 2018 Mar 5;150(3):401-415. doi: 10.1085/jgp.201711938. Epub 2018 Jan 26. J Gen Physiol. 2018. PMID: 29374022 Free PMC article.
-
Dissecting the determinants of light sensitivity in amphioxus microvillar photoreceptors: possible evolutionary implications for melanopsin signaling.J Neurosci. 2012 Dec 12;32(50):17977-87. doi: 10.1523/JNEUROSCI.3069-12.2012. J Neurosci. 2012. PMID: 23238714 Free PMC article.

