A peptide-binding motif for I-A(g7), the class II major histocompatibility complex (MHC) molecule of NOD and Biozzi AB/H mice
- PMID: 9091575
- PMCID: PMC2196246
- DOI: 10.1084/jem.185.6.1013
A peptide-binding motif for I-A(g7), the class II major histocompatibility complex (MHC) molecule of NOD and Biozzi AB/H mice
Abstract
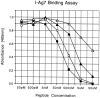
The class II major histocompatibility complex molecule I-A(g7) is strongly linked to the development of spontaneous insulin-dependent diabetes mellitus (IDDM) in non obese diabetic mice and to the induction of experimental allergic encephalomyelitis in Biozzi AB/H mice. Structurally, it resembles the HLA-DQ molecules associated with human IDDM, in having a non-Asp residue at position 57 in its beta chain. To identify the requirements for peptide binding to I-A(g7) and thereby potentially pathogenic T cell epitopes, we analyzed a known I-A(g7)-restricted T cell epitope, hen egg white lysozyme (HEL) amino acids 9-27. NH2- and COOH-terminal truncations demonstrated that the minimal epitope for activation of the T cell hybridoma 2D12.1 was M12-R21 and the minimum sequence for direct binding to purified I-A(g7) M12-Y20/K13-R21. Alanine (A) scanning revealed two primary anchors for binding at relative positions (p) 6 (L) and 9 (Y) in the HEL epitope. The critical role of both anchors was demonstrated by incorporating L and Y in poly(A) backbones at the same relative positions as in the HEL epitope. Well-tolerated, weakly tolerated, and nontolerated residues were identified by analyzing the binding of peptides containing multiple substitutions at individual positions. Optimally, p6 was a large, hydrophobic residue (L, I, V, M), whereas p9 was aromatic and hydrophobic (Y or F) or positively charged (K, R). Specific residues were not tolerated at these and some other positions. A motif for binding to I-A(g7) deduced from analysis of the model HEL epitope was present in 27/30 (90%) of peptides reported to be I-A(g7)-restricted T cell epitopes or eluted from I-A(g7). Scanning a set of overlapping peptides encompassing human proinsulin revealed the motif in 6/6 good binders (sensitivity = 100%) and 4/13 weak or non-binders (specificity = 70%). This motif should facilitate identification of autoantigenic epitopes relevant to the pathogenesis and immunotherapy of IDDM.
Figures

References
-
- Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. - PubMed
-
- Liu GY, Baker D, Fairchild S, Figueroa F, QuarteyPapafio R, Tone M, Healey D, Cooke A, Turk JL, Wraith DC. Complete characterization of the expressed immune response genes in Biozzi AB/H mice: structural and functional identity between AB/H and NOD A region molecules. Immunogenetics. 1993;37:296–300. - PubMed
-
- Tait BD, Harrison LC. Overview: the major histocompatibility complex and insulin-dependent diabetes mellitus. Baillieres Clin Endocrinol & Metab. 1991;5:211–228. - PubMed
-
- Rammensee H-G. Chemistry of peptides associated with MHC class I and class II molecules. Curr Opin Immunol. 1995;7:85–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

