An HLA-DR1 transgene confers susceptibility to collagen-induced arthritis elicited with human type II collagen
- PMID: 9091584
- PMCID: PMC2196244
- DOI: 10.1084/jem.185.6.1113
An HLA-DR1 transgene confers susceptibility to collagen-induced arthritis elicited with human type II collagen
Abstract
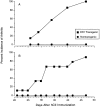
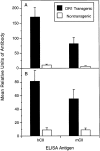
Rheumatoid arthritis (RA) is an autoimmune disease that is strongly associated with the expression of several HLA-DR haplotypes, including DR1 (DRB1*0101). Although the antigen that initiates RA remains elusive, it has been shown that many patients have autoimmunity directed to type II collagen (CII). To test the hypothesis that HLA-DR1 is capable of mediating an immune response to CII, we have generated transgenic mice expressing chimeric (human/mouse) HLA-DR1. When the DR1 transgenic mice were immunized with human CII (hCII), they developed a severe autoimmune arthritis, evidenced by severe swelling and erythema of the limbs and marked inflammation and erosion of articular joints. The development of the autoimmune arthritis was accompanied by strong DR1-restricted T and B cell responses to hCII. The T cell response was focused on a dominant determinant contained within CII(259-273) from which an eight amino acid core was defined. The B cell response was characterized by high titers of antibody specific for hCII, and a high degree of cross-reactivity with murine type II collagen. These data demonstrate that HLA-DR1 is capable of presenting peptides derived from hCII, and suggest that this DR1 transgenic model will be useful in the development of DR1-specific therapies for RA.
Figures




References
-
- Stastny P, Ball EJ, Dry PJ, Nunez G. The human immune response region (HLA-D) and disease susceptibility. Immunol Rev. 1983;70:113–154. - PubMed
-
- Stastny P, Ball E, Kahn M, Olsen N, Pincus T, Gao X. HLA-DR4 and other genetic markers in rheumatoid arthritis. Br J Rheumatol. 1988;27:132–138. - PubMed
-
- Nepom GT, Byers P, Seyfried C, Healey LA, Wilske KR, Stage D, Nepom BS. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989;32:15–21. - PubMed
-
- Gregersen P, Silver J, Winchester R. The shared epitope hypothesis: an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

