K+ channel expression and cell proliferation are regulated by intracellular sodium and membrane depolarization in oligodendrocyte progenitor cells
- PMID: 9092588
- PMCID: PMC6573116
- DOI: 10.1523/JNEUROSCI.17-08-02669.1997
K+ channel expression and cell proliferation are regulated by intracellular sodium and membrane depolarization in oligodendrocyte progenitor cells
Abstract
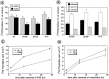
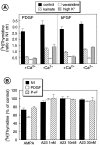
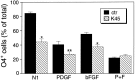
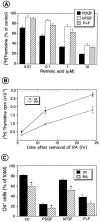
The effects of a variety of antiproliferative agents on voltage-dependent K+ channel function in cortical oligodendrocyte progenitor (O-2A) cells were studied. Previously, we had shown that glutamate receptor activation reversibly inhibited O-2A cell proliferation stimulated by mitogenic factors and prevented lineage progression by attenuating outward K+ currents in O-2A cells. We now show that the antiproliferative actions of glutamate receptor activation are Ca2+-independent and arise from an increase in intracellular Na+ and subsequent block of outward K+ currents. In support of this mechanism, agents that acted to depolarize O-2A cells or increase intracellular sodium similarly had an antiproliferative effect, attributable at least in part to a reduction in voltage-gated K+ currents. Also, these effects were reversible and Ca2+-independent. Chronic treatment with glutamate agonists was without any long-term effect on K+ current function. Cells cultured in elevated K+, however, demonstrated an upregulation of inward rectifier K+ currents, concomitant with an hyperpolarization of the resting membrane potential. This culture condition therefore promoted a current phenotype typical of pro-oligodendroblasts. Finally, cells chronically treated with the mitotic inhibitor retinoic acid displayed a selective downregulation of outward K+ currents. In conclusion, signals that affect O-2A cell proliferation do so by regulating K+ channel function. These data indicate that the regulation of K+ currents in cells of the oligodendrocyte lineage plays an important role in determining their proliferative potential and demonstrate that O-2A cell K+ current phenotype can be modified by long-term depolarization of the cell membrane.
Figures






References
-
- Armstrong RC, Harvath L, Dubois-Dalcq M. Type 1 astrocytes and oligodendrocytes-type 2 astrocytes glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–407. - PubMed
-
- Barres BA, Chun LLY, Corey DP. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron. 1989;2:1375–1388. - PubMed
-
- Barres BA, Koroshetz WJ, Swartz KJ, Chun LLY, Corey DP. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990;4:507–524. - PubMed
-
- Barres BA, Schmid R, Sendtner M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. - PubMed
-
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
