Signal transduction mediated by the truncated trkB receptor isoforms, trkB.T1 and trkB.T2
- PMID: 9092589
- PMCID: PMC6573096
- DOI: 10.1523/JNEUROSCI.17-08-02683.1997
Signal transduction mediated by the truncated trkB receptor isoforms, trkB.T1 and trkB.T2
Abstract
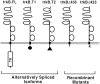
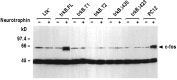
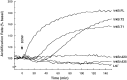
The trkB family of transmembrane proteins serves as receptors for BDNF and NT-4/5. The family is composed of a tyrosine kinase-containing isoform as well as several alternatively spliced "truncated receptors" with identical extracellular ligand-binding domains but very small intracellular domains. The two best-characterized truncated trkB receptors, designated as trkB.T1 and trkB.T2, contain intracellular domains of only 23 and 21 amino acids, respectively. Although it is known that the tyrosine kinase isoform (trkB.FL) is capable of initiating BDNF and NT-4/5-induced signal transduction, the functional role or roles of the truncated receptors remain enigmatic. At the same time, the potential importance of the truncated receptors in the development, maintenance, and regeneration of the nervous system has been highlighted by recent developmental and injury paradigm investigations. Here we have used trkB cDNA transfected cell lines to demonstrate that both trkB.T1 and trkB.T2 are capable of mediating BDNF-induced signal transduction. More specifically, BDNF activation of either trkB.T1 or trkB.T2 increases the rate of acidic metabolite release from the cell, a common physiological consequence of many signaling pathways. Further, these trkB.T1- and trkB. T2-mediated changes occur with kinetics distinct from changes mediated by trkB.FL, suggesting the participation of at least some unique rate-limiting component or components. Mutational analysis demonstrates that the isoform-specific sequences within the intracellular domains of each receptor are essential for signaling capability. Finally, inhibitor studies suggest that kinases are likely to be involved in the trkB.T1 and trkB.T2 signaling pathways.
Figures

References
-
- Armanini MP, McMahon SB, Sutherland J, Shelton DL, Philips HS. Truncated and catalytic isoforms of trkB are co-expressed in neurons of rat and mouse CNS. Eur J Neurosci. 1995;7:1403–1409. - PubMed
-
- Baxter GT, Miller DL, Kuo RC, Wada HG, Owicki JC. PKC epsilon is involved in granulocyte-macrophage colony-stimulating factor signal transduction: evidence from microphysiometry and antisense oligonucleotide experiments. Biochemistry. 1992;31:10950–10954. - PubMed
-
- Baxter GT, Young ML, Miller DL, Owicki JC. Using microphysiometry to study the pharmacology of exogenously expressed m1 and m3 muscarinic receptors. Life Sci. 1994;55:573–583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
