Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria
- PMID: 9096383
- PMCID: PMC20359
- DOI: 10.1073/pnas.94.7.3274
Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria
Abstract
Expression of molecules with antiparasitic activity by genetically transformed symbiotic bacteria of disease-transmitting insects may serve as a powerful approach to control certain arthropod-borne diseases. The endosymbiont of the Chagas disease vector, Rhodnius prolixus, has been transformed to express cecropin A, a peptide lethal to the parasite, Trypanosoma cruzi. In insects carrying the transformed bacteria, cecropin A expression results in elimination or reduction in number of T. cruzi. A method has been devised to spread the transgenic bacteria to populations of R. prolixus, in a manner that mimics their natural coprophagous route of symbiont acquisition.
Figures




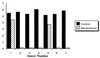
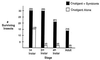
References
-
- Georghiou, G. P. (1990) in Overview of Insecticide Resistance to Agrochemicals, American Chemical Society Symposium Series, eds. Green, M. B., LeBaron, H. M. & Moberg, W. K. (Am. Chem. Soc., Washington, DC).
-
- Georghiou G P. Pesticide Resistance Strategies and Tactics for Management. National Research Council; Academic, New York: Board on Agriculture; 1986. pp. 11–14.
-
- Mogi M, Teji S. Advances in Disease Vector Research. New York: Springer; 1991. pp. 47–76.
-
- Dasch G A, Weiss E, Chang K. Bergey’s Manual of Systematic Bacteriology. Vol. 1. Baltimore: Williams & Wilkins; 1984. pp. 811–833.
-
- Olson K E, Higgs S, Gaines P J, Powers A M, Davis B S, Kamrud K I, Carlson J O, Blair C D, Beaty B J. Science. 1996;272:884–886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

