Peptide-induced nasal tolerance for a mycobacterial heat shock protein 60 T cell epitope in rats suppresses both adjuvant arthritis and nonmicrobially induced experimental arthritis
- PMID: 9096385
- PMCID: PMC20361
- DOI: 10.1073/pnas.94.7.3284
Peptide-induced nasal tolerance for a mycobacterial heat shock protein 60 T cell epitope in rats suppresses both adjuvant arthritis and nonmicrobially induced experimental arthritis
Abstract
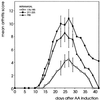
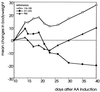
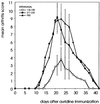
Adjuvant arthritis (AA) can be induced in Lewis rats by immunization with mycobacterial antigens. Passive transfer of a T cell clone recognizing the 180-188 amino acid sequence in mycobacterial heat shock protein 60 (hsp60) was found to induce AA. In the present study, we investigated whether tolerance was obtained for this AA-associated T cell epitope after intranasal or s.c. administration of a peptide containing this epitope. Two 15-mer peptides containing the mycobacterial hsp60 sequences 176-190 and 211-225 were used; 176-190 contained the T cell epitope 180-188, which was recognized by the arthritogenic T cell clone A2b and was the immunodominant hsp60 T cell epitope after induction of AA, and 211-225 contained a T cell epitope that was recognized both after induction of arthritis with whole Mycobacterium tuberculosis and after immunization with mycobacterial hsp60. In rats treated intranasally or subcutaneously with 176-190 and immunized with mycobacterial hsp60, proliferative responses to 176-190 were reduced. Proliferative responses to 211-225 and to whole mycobacterial hsp60 were not affected. AA was inhibited intranasally in the 176-190-treated rats but not in the 211-225-treated rats. Moreover, intranasal 176-190 led to similar arthritis-protective effects in a nonmicrobially induced experimental arthritis (avridine-induced arthritis). Therefore, tolerance for a disease-triggering, microbial cartilage-mimicking epitope may cause resistance to arthritis irrespective of the actual trigger leading to development of the disease.
Figures



Similar articles
-
Nasal administration of arthritis-related T cell epitopes of heat shock protein 60 as a promising way for immunotherapy in chronic arthritis.Biotherapy. 1998;10(3):205-11. doi: 10.1007/BF02678298. Biotherapy. 1998. PMID: 9559975 Review.
-
Heat-shock protein T-cell epitopes trigger a spreading regulatory control in a diversified arthritogenic T-cell response.Immunol Rev. 1998 Aug;164:169-74. doi: 10.1111/j.1600-065x.1998.tb01218.x. Immunol Rev. 1998. PMID: 9795774 Review.
-
Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis.J Exp Med. 1995 Mar 1;181(3):943-52. doi: 10.1084/jem.181.3.943. J Exp Med. 1995. PMID: 7869052 Free PMC article.
-
T cell reactivity to an epitope of the mycobacterial 65-kDa heat-shock protein (hsp 65) corresponds with arthritis susceptibility in rats and is regulated by hsp 65-specific cellular responses.Eur J Immunol. 1991 May;21(5):1289-96. doi: 10.1002/eji.1830210529. Eur J Immunol. 1991. PMID: 1709871
-
Differential mycobacterial 65-kDa heat shock protein T cell epitope recognition after adjuvant arthritis-inducing or protective immunization protocols.J Immunol. 1994 Apr 1;152(7):3656-64. J Immunol. 1994. PMID: 8144941
Cited by
-
A peptide derived from HSP60 reduces proinflammatory cytokines and soluble mediators: a therapeutic approach to inflammation.Front Immunol. 2023 Apr 28;14:1162739. doi: 10.3389/fimmu.2023.1162739. eCollection 2023. Front Immunol. 2023. PMID: 37187739 Free PMC article. Review.
-
Successful immunotherapy with matrix metalloproteinase-derived peptides in adjuvant arthritis depends on the timing of peptide administration.Arthritis Res. 2002;4(4):R2. doi: 10.1186/ar421. Epub 2002 May 7. Arthritis Res. 2002. PMID: 12106501 Free PMC article.
-
Regulation of peripheral inflammation by spinal p38 MAP kinase in rats.PLoS Med. 2006 Sep;3(9):e338. doi: 10.1371/journal.pmed.0030338. PLoS Med. 2006. PMID: 16953659 Free PMC article.
-
The gene expression profile of preclinical autoimmune arthritis and its modulation by a tolerogenic disease-protective antigenic challenge.Arthritis Res Ther. 2011;13(5):R143. doi: 10.1186/ar3457. Epub 2011 Sep 13. Arthritis Res Ther. 2011. PMID: 21914168 Free PMC article.
-
Oral tolerance and the treatment of rheumatoid arthritis.Springer Semin Immunopathol. 1998;20(1-2):289-308. doi: 10.1007/BF00832013. Springer Semin Immunopathol. 1998. PMID: 9836383 Review. No abstract available.
References
-
- Whitehouse D J, Whitehouse M W, Pearson C M. Nature (London) 1969;224:1322–1326. - PubMed
-
- Holoshitz J, Naparstek Y, Ben-Nun A, Cohen I R. Science. 1983;219:56–58. - PubMed
-
- Van Eden W, Thole J E R, van der Zee R, Noordzij A, van Embden J D A, Hensen E J, Cohen I R. Nature (London) 1988;331:171–173. - PubMed
-
- Higgins P J, Weiner H L. J Immunol. 1988;140:440–445. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

