HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses
- PMID: 9096397
- PMCID: PMC20373
- DOI: 10.1073/pnas.94.7.3352
HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses
Abstract
The subgroup C of the adenoviruses (Ad) and the group B coxsackieviruses (CVB) are structurally unrelated viruses that are known to compete for an unidentified cell surface receptor. We now describe the isolation of cDNAs from human and mouse that encode the human CVB and Ad2 and 5 receptor (HCAR) and the mouse CVB Ad2 and 5 receptor (MCAR). Both are 46-kDa glycoproteins whose primary amino acid sequences are highly homologous. Structurally, HCAR and MCAR appear to be transmembrane proteins that contain two extracellular immunoglobulin-like domains and therefore belong to this superfamily. Transfection of either of these cDNA molecules into receptor-negative NIH 3T3 cells conferred susceptibility to CVB infection and permitted the expression of beta-galactosidase from a recombinant Ad5 vector. In addition, HCAR and MCAR mRNAs could be detected on Northern blots of oligo(dT)-selected RNA from receptor-positive HeLa cells and TCMK-1 as well as several tissues of human and mouse origin that are known to be targets for Ad and CVB infections. Finally, Western blots using antibodies that inhibit virus binding to either the human or mouse CVB receptors detected 46-kDa proteins in HCAR- and MCAR-transfected cells, respectively. Taken together, these results confirm that the isolated cDNAs encode the receptors for the subgroup C Ad and CVB.
Figures
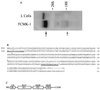

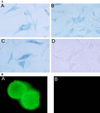

References
-
- Lonberg-Holm K, Crowell R L, Philipson L. Nature (London) 1976;259:679–681. - PubMed
-
- Xia D, Henry L J, Gerard R D, Deisenhofer J. Structure (London) 1994;2:1259–1270. - PubMed
-
- Muckelbauer J K, Kremer M, Minor I, Diana G, Dutko F J, Groarke J, Pevear D C, Rossman M G. Structure (London) 1995;3:653–667. - PubMed
-
- Shenk T. In: Virology. Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath J P, Roizman B, Straus S E, editors. Philadelphia: Lippincott-Raven; 1996. pp. 2112–2137.
-
- Melnick J L. In: Virology. Fields B N, Knipe D M, Chanock R M, Hirsch M S, Melnick J L, Monath J P, editors. New York: Raven; 1990. pp. 549–600.
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

