Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa
- PMID: 9096399
- PMCID: PMC20375
- DOI: 10.1073/pnas.94.7.3363
Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa
Abstract
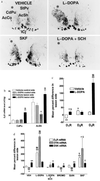
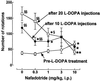
In rats with unilateral lesions of the nigrostriatal dopamine pathway with 6-hydroxydopamine, the motor stimulating effects of levodopa, an indirect dopamine receptor agonist, evidenced by contraversive rotations, become enhanced upon repeated intermittent administration. However, the mechanisms of this behavioral sensitization are essentially unknown. We show that development of sensitization is accompanied by a progressive appearance of D3 receptor mRNA and binding sites, visualized by in situ hybridization and 7-[3H] hydroxy-N,N-di-n-propyl-2-aminotetralin autoradiography, respectively, occurring in the denervated caudate putamen, a brain area from which this receptor subtype is normally absent. Development and decay of these two processes occur with closely parallel time courses, whereas there were no marked changes in D1 or D2 receptor mRNAs. D3 receptor induction by levodopa is mediated by repeated D1 receptor stimulation, since it is prevented by the antagonist SCH 33390 and mimicked by the agonist SKF 38393, but not by two D2 receptor agonists. The enhanced behavioral response to levodopa is mediated by the newly synthesized D3 receptor, since it is antagonized by nafadotride, a preferential D3 receptor antagonist, in low dosage, which has no such effect before D3 receptor induction. D3 receptor induction and behavioral sensitization are also accompanied by a sustained enhancement of prodynorphin mRNA level and a progressively decreasing expression of the preprotachykinin gene. We propose that imbalance between dynorphin and substance P release from the same striatonigral motor efferent pathway, related to D3 receptor induction, is responsible for behavioral sensitization.
Figures


References
-
- Robinson T E, Becker J B. Brain Res Rev. 1986;11:157–198. - PubMed
-
- Kalivas P W, Stewart J. Brain Res Rev. 1991;16:223–244. - PubMed
-
- Hornykiewicz O. Pharmacol Rev. 1966;18:925–964. - PubMed
-
- Hardie R J. In: Parkinson’s Disease. Stern G M, editor. Baltimore: The Johns Hopkins Univ. Press; 1990. pp. 559–596.
-
- Gancher S T. In: Parkinson’s Disease Neurobehavioral Aspects. Huber S T, Cummings J L, editors. New York: Oxford Univ. Press; 1992. pp. 273–287.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

