Multi-functional acetyl-CoA carboxylase from Brassica napus is encoded by a multi-gene family: indication for plastidic localization of at least one isoform
- PMID: 9096417
- PMCID: PMC20393
- DOI: 10.1073/pnas.94.7.3465
Multi-functional acetyl-CoA carboxylase from Brassica napus is encoded by a multi-gene family: indication for plastidic localization of at least one isoform
Abstract
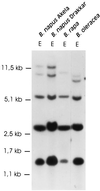
Three genes coding for different multifunctional acetyl-CoA carboxylase (ACCase; EC 6.4.1.2) isoenzymes from Brassica napus were isolated and divided into two major classes according to structural features in their 5' regions: class I comprises two genes with an additional coding exon of approximately 300 bp at the 5' end, and class II is represented by one gene carrying an intron of 586 bp in its 5' untranslated region. Fusion of the peptide sequence encoded by the additional first exon of a class I ACCase gene to the jellyfish Aequorea victoria green fluorescent protein (GFP) and transient expression in tobacco protoplasts targeted GFP to the chloroplasts. In contrast to the deduced primary structure of the biotin carboxylase domain encoded by the class I gene, the corresponding amino acid sequence of the class II ACCase shows higher identity with that of the Arabidopsis ACCase, both lacking a transit peptide. The Arabidopsis ACCase has been proposed to be a cytosolic isoenzyme. These observations indicate that the two classes of ACCase genes encode plastidic and cytosolic isoforms of multi-functional, eukaryotic type, respectively, and that B. napus contains at least one multi-functional ACCase besides the multi-subunit, prokaryotic type located in plastids. Southern blot analysis of genomic DNA from B. napus, Brassica rapa, and Brassica oleracea, the ancestors of amphidiploid rapeseed, using a fragment of a multi-functional ACCase gene as a probe revealed that ACCase is encoded by a multi-gene family of at least five members.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

