Role of tyrosine phosphorylation of HS1 in B cell antigen receptor-mediated apoptosis
- PMID: 9104825
- PMCID: PMC2196252
- DOI: 10.1084/jem.185.7.1387
Role of tyrosine phosphorylation of HS1 in B cell antigen receptor-mediated apoptosis
Abstract
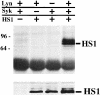
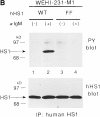
The 75-kD HS1 protein is highly tyrosine-phosphorylated during B cell antigen receptor (BCR)-mediated signaling. Owing to low expression of HS1, WEHI-231-derived M1 cells, unlike the parental cells, are insensitive to BCR-mediated apoptosis. Here, we show that BCR-associated tyrosine kinases Lyn and Syk synergistically phosphorylate HS1, and that Tyr-378 and Tyr-397 of HS1 are the critical residues for its BCR-induced phosphorylation. In addition, unlike wild-type HS1, a mutant HS1 carrying the mutations Phe-378 and Phe-397 was unable to render M1 cells sensitive to apoptosis. Wild-type HS1, but not the mutant, localized to the nucleus under the synergy of Lyn and Syk. Thus, tyrosine phosphorylation of HS1 is required for BCR-induced apoptosis and nuclear translocation of HS1 may be a prerequisite for B cell apoptosis.
Figures





References
-
- Yamanashi Y, Kakiuchi T, Mizuguchi J, Yamamoto T, Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science (Wash DC) 1991;251:192–194. - PubMed
-
- Bolen JB. Protein tyrosine kinases in the initiation of antigen receptor signaling. Curr Opin Immunol. 1995;7:306–311. - PubMed
-
- Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. - PubMed
-
- Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

