Ii chain controls the transport of major histocompatibility complex class II molecules to and from lysosomes
- PMID: 9105036
- PMCID: PMC2139866
- DOI: 10.1083/jcb.137.1.51
Ii chain controls the transport of major histocompatibility complex class II molecules to and from lysosomes
Abstract
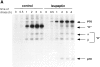
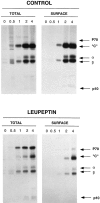
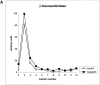
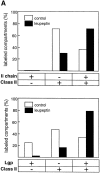
Major histocompatibility complex class II molecules are synthesized as a nonameric complex consisting of three alpha beta dimers associated with a trimer of invariant (Ii) chains. After exiting the TGN, a targeting signal in the Ii chain cytoplasmic domain directs the complex to endosomes where Ii chain is proteolytically processed and removed, allowing class II molecules to bind antigenic peptides before reaching the cell surface. Ii chain dissociation and peptide binding are thought to occur in one or more postendosomal sites related either to endosomes (designated CIIV) or to lysosomes (designated MIIC). We now find that in addition to initially targeting alpha beta dimers to endosomes, Ii chain regulates the subsequent transport of class II molecules. Under normal conditions, murine A20 B cells transport all of their newly synthesized class II I-A(b) alpha beta dimers to the plasma membrane with little if any reaching lysosomal compartments. Inhibition of Ii processing by the cysteine/serine protease inhibitor leupeptin, however, blocked transport to the cell surface and caused a dramatic but selective accumulation of I-A(b) class II molecules in lysosomes. In leupeptin, I-A(b) dimers formed stable complexes with a 10-kD NH2-terminal Ii chain fragment (Ii-p10), normally a transient intermediate in Ii chain processing. Upon removal of leupeptin, Ii-p10 was degraded and released, I-A(b) dimers bound antigenic peptides, and the peptide-loaded dimers were transported slowly from lysosomes to the plasma membrane. Our results suggest that alterations in the rate or efficiency of Ii chain processing can alter the postendosomal sorting of class II molecules, resulting in the increased accumulation of alpha beta dimers in lysosome-like MIIC. Thus, simple differences in Ii chain processing may account for the highly variable amounts of class II found in lysosomal compartments of different cell types or at different developmental stages.
Figures















References
-
- Amigorena S, Drake JR, Webster P, Mellman I. Transient accumulation of new class II molecules in a novel endocytic compartment in B lymphocytes. Nature (Lond) 1994;369:113–120. - PubMed
-
- Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

