Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells
- PMID: 9105039
- PMCID: PMC2139854
- DOI: 10.1083/jcb.137.1.93
Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells
Abstract
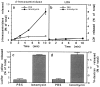
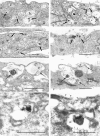
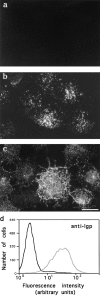
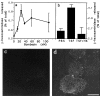
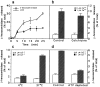
Lysosomes are considered to be a terminal degradative compartment of the endocytic pathway, into which transport is mostly unidirectional. However, specialized secretory vesicles regulated by Ca2+, such as neutrophil azurophil granules, mast cell-specific granules, and cytotoxic lymphocyte lytic granules, share characteristics with lysosomes that may reflect a common biogenesis. In addition, the involvement of Ca2+ transients in the invasion mechanism of the parasite Trypanosoma cruzi, which occurs by fusion of lysosomes with the plasma membrane, suggested that lysosome exocytosis might be a generalized process present in most cell types. Here we demonstrate that elevation in the intracellular free Ca2+ concentration of normal rat kidney (NRK) fibroblasts induces fusion of lysosomes with the plasma membrane. This was verified by measuring the release of the lysosomal enzyme beta-hexosaminidase, the appearance on the plasma membrane of the lysosomal glycoprotein lgp120, the release of fluid-phase tracers previously loaded into lysosomes, and the release of the lysosomally processed form of cathepsin D. Exposure to the Ca2+ ionophore ionomycin or addition of Ca2+-containing buffers to streptolysin O-permeabilized cells induced exocytosis of approximately 10% of the total lysosomes of NRK cells. The process was also detected in other cell types such as epithelial cells and myoblasts. Lysosomal exocytosis was found to require micromolar levels of Ca2+ and to be temperature and ATP dependent, similar to Ca2+-regulated secretory mechanisms in specialized cells. These findings highlight a novel role for lysosomes in cellular membrane traffic and suggest that fusion of lysosomes with the plasma membrane may be an ubiquitous form of Ca2+-regulated exocytosis.
Figures
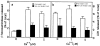
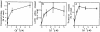

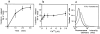
References
-
- Ahnert-Hilger G, Mach W, Fohr KJ, Gratzl M. Poration by α-toxin and streptolysin-O: an approach to analyze intracellular processes. Methods Cell Biol. 1989;31:63–90. - PubMed
-
- Baron R, Neff L, Brown W, Louvard D, Courtnoy PJ. Selective internalization of the apical plasma membrane and rapid redistribution of lysosomal enzymes and mannose-6-phosphate receptors during osteoclast inactivation by calcitonin. J Cell Sci. 1990;97:439–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

