Point mutations in human beta cardiac myosin heavy chain have differential effects on sarcomeric structure and assembly: an ATP binding site change disrupts both thick and thin filaments, whereas hypertrophic cardiomyopathy mutations display normal assembly
- PMID: 9105042
- PMCID: PMC2139848
- DOI: 10.1083/jcb.137.1.131
Point mutations in human beta cardiac myosin heavy chain have differential effects on sarcomeric structure and assembly: an ATP binding site change disrupts both thick and thin filaments, whereas hypertrophic cardiomyopathy mutations display normal assembly
Abstract
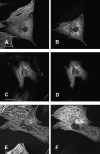
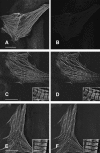
Hypertrophic cardiomyopathy is a human heart disease characterized by increased ventricular mass, focal areas of fibrosis, myocyte, and myofibrillar disorganization. This genetically dominant disease can be caused by mutations in any one of several contractile proteins, including beta cardiac myosin heavy chain (beta MHC). To determine whether point mutations in human beta MHC have direct effects on interfering with filament assembly and sarcomeric structure, full-length wild-type and mutant human beta MHC cDNAs were cloned and expressed in primary cultures of neonatal rat ventricular cardiomyocytes (NRC) under conditions that promote myofibrillogenesis. A lysine to arginine change at amino acid 184 in the consensus ATP binding sequence of human beta MHC resulted in abnormal subcellular localization and disrupted both thick and thin filament structure in transfected NRC. Diffuse beta MHC K184R protein appeared to colocalize with actin throughout the myocyte, suggesting a tight interaction of these two proteins. Human beta MHC with S472V mutation assembled normally into thick filaments and did not affect sarcomeric structure. Two mutant myosins previously described as causing human hypertrophic cardiomyopathy, R249Q and R403Q, were competent to assemble into thick filaments producing myofibrils with well defined I bands, A bands, and H zones. Coexpression and detection of wild-type beta MHC and either R249Q or R403Q proteins in the same myocyte showed these proteins are equally able to assemble into the sarcomere and provided no discernible differences in subcellular localization. Thus, human beta MHC R249Q and R403Q mutant proteins were readily incorporated into NRC sarcomeres and did not disrupt myofilament formation. This study indicates that the phenotype of myofibrillar disarray seen in HCM patients which harbor either of these two mutations may not be directly due to the failure of the mutant myosin heavy chain protein to assemble and form normal sarcomeres, but may rather be a secondary effect possibly resulting from the chronic stress of decreased beta MHC function.
Figures






References
-
- Beall CJ, Sepanski MA, Fyrberg EA. Genetic dissection of Drosophilamyofibril formation: effects of actin and myosin heavy chain null alleles. Genes Dev. 1989;3:131–140. - PubMed
-
- Bejsovec A, Anderson P. Myosin heavy-chain mutations that disrupt Caenorhabditis elegansthick filament assembly. Genes Dev. 1988;2:1307–1317. - PubMed
-
- Bejsovec A, Anderson P. Functions of the myosin ATP and actin binding sites are required for C. elegansthick filament assembly. Cell. 1990;60:133–140. - PubMed
-
- Bonne G, Carrier L, Bercovici J, Cruaud C, Richard P, Hainque B, Gautel M, Labeit S, James M, Beckmann J, et al. Cardiac myosin binding protein-C gene splice acceptor site mutation is associated with familial hypertrophic cardiomyopathy. Nat Genet. 1995;11:438–440. - PubMed
-
- Chen CA, Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotech. 1988;6:632–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

