Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies
- PMID: 9105051
- PMCID: PMC2139858
- DOI: 10.1083/jcb.137.1.231
Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies
Abstract
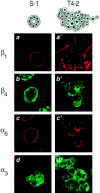
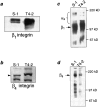
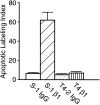
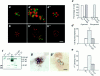
In a recently developed human breast cancer model, treatment of tumor cells in a 3-dimensional culture with inhibitory beta1-integrin antibody or its Fab fragments led to a striking morphological and functional reversion to a normal phenotype. A stimulatory beta1-integrin antibody proved to be ineffective. The newly formed reverted acini re-assembled a basement membrane and re-established E-cadherin-catenin complexes, and re-organized their cytoskeletons. At the same time they downregulated cyclin D1, upregulated p21(cip,wat-1), and stopped growing. Tumor cells treated with the same antibody and injected into nude mice had significantly reduced number and size of tumors in nude mice. The tissue distribution of other integrins was also normalized, suggesting the existence of intimate interactions between the different integrin pathways as well as adherens junctions. On the other hand, nonmalignant cells when treated with either alpha6 or beta4 function altering antibodies continued to grow, and had disorganized colony morphologies resembling the untreated tumor colonies. This shows a significant role of the alpha6/beta4 heterodimer in directing polarity and tissue structure. The observed phenotypes were reversible when the cells were disassociated and the antibodies removed. Our results illustrate that the extracellular matrix and its receptors dictate the phenotype of mammary epithelial cells, and thus in this model system the tissue phenotype is dominant over the cellular genotype.
Figures



References
-
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. - PubMed
-
- Alford D, Taylor-Papadimitriou J. Cell adhesion molecules in the normal and cancerous mammary gland. J Mamm Gland Biol Neo. 1996;1:207–218. - PubMed
-
- Barsky, S.H., S.A. Doberneck, D.A. Grossman, and S.M. Love. 1994. Recapitulation of carcinoma in situ in axillary nodal breast carcinoma metastasis: clinical and biological implications. Breast Cancer Res.Treat. 32(Suppl): 8.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

