Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase
- PMID: 9108028
- PMCID: PMC20491
- DOI: 10.1073/pnas.94.8.3627
Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase
Abstract
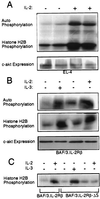
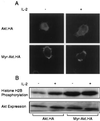
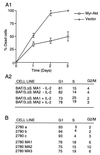
The interleukin-2 (IL-2) receptor (IL-2R) is composed of three subunits. Of these, IL-2Ra is required for high-affinity IL-2 binding, while IL-2R beta and IL-2R gamma(c) are required for the transduction of IL-2-generated signals. Signals transduced via the S region of the IL-2R beta (amino acids 267-322) in BAF/3 cells activate the phosphatidylinositol 3-kinase (PI3-kinase) and induce the expression of Bcl-2 and c-myc. Through the induction of Bcl-2, IL-2 inhibits apoptosis and through the combination of Bcl-2 and c-myc it stimulates progression through the cell cycle. Here we show that the protein kinase encoded by the Akt proto-oncogene is activated by IL-2. Akt activation by IL-2 depends on PI3-kinase signals transduced via the S region of the IL-2R beta and is linked to the translocation of Akt to the cell membrane. Expression of catalytically active Akt mutants in BAF/3 cells expressing IL-2R beta[A0]delta S promotes the expression of Bcl-2 and c-myc, inhibits apoptosis induced by IL-3 deprivation or staurosporine, and stimulates cell cycle progression. The same mutants also stimulate cell cycle progression in 2780a, an IL-2-dependent T cell line that undergoes G1 arrest rather than apoptosis after IL-2 deprivation. The activation of Akt by IL-2 via the PI3-kinase and the rescue of the PI3-kinase-mediated antiapoptotic and proliferative IL-2 signals by catalytically active Akt indicate that these signals are transduced by Akt.
Figures


References
-
- Taniguchi T, Miyazaki T, Minami Y, Kawahara A, Fujii H, Nakagawa Y, Hatakeyama M, Liu Z J. Ann NY Acad Sci. 1995;766:235–244. - PubMed
-
- Ihle J N, Witthuhn B A, Quelle F W, Yamamoto K, Silvennoinen O. Annu Rev Immunol. 1995;13:369–398. - PubMed
-
- Sagamura K, Asao H, Kondo M, Tanaka N, Ishii N, Nakamura M, Takeshita T. Adv Immunol. 1995;59:225–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

