AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1
- PMID: 9108029
- PMCID: PMC20492
- DOI: 10.1073/pnas.94.8.3633
AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1
Abstract
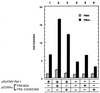
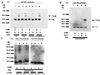
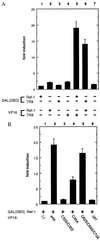
Thioredoxin (TRX) is a pleiotropic cellular factor that has thiol-mediated redox activity and is important in regulation of cellular processes, including proliferation, apoptosis, and gene expression. The activity of several transcription factors is posttranslationally altered by redox modification(s) of specific cysteine residue(s). One such factor is nuclear factor (NF)-kappa B, whose DNA-binding activity is markedly augmented by TRX treatment in vitro. Similarly, the DNA-binding activity of activator protein 1 (AP-1) is modified by a DNA repair enzyme, redox factor 1 (Ref-1), which is identical to a DNA repair enzyme, AP endonuclease. Ref-1 activity is in turn modulated by various redox-active compounds, including TRX. We here report the molecular cascade of redox regulation of AP-1 mediated by TRX and Ref-1. Phorbol 12-myristate 13 acetate efficiently translocated TRX into the HeLa cell nucleus where Ref-1 preexists. This process seems to be essential for AP-1 activation by redox modification because co-overexpression of TRX and Ref-1 in COS-7 cells potentiated AP-1 activity only after TRX was transported into the nucleus by phorbol 12-myristate 13 acetate treatment. To prove the direct active site-mediated association between TRX and Ref-1, we generated a series of substitution-mutant cysteine residues of TRX. In both an in vitro diamide-induced cross-linking study and an in vivo mammalian two-hybrid assay we proved that TRX can associate directly with Ref-1 in the nucleus; also, we demonstrated the requirement of cysteine residues in the TRX catalytic center for the potentiation of AP-1 activity. This report presents an example of a cascade in cellular redox regulation.
Figures


References
-
- Pahl H L, Baeuerle P A. BioEssays. 1994;16:497–502. - PubMed
-
- Laurent T C, Moore E C, Reichard P. J Biol Chem. 1964;239:3436–3444. - PubMed
-
- Holmgren A. Annu Rev Biochem. 1985;54:237–271. - PubMed
-
- Holmgren A. J Biol Chem. 1989;264:13963–13966. - PubMed
-
- Buchanan B B, Schurmann P, Decottignies P, Lozano R M. Arch Biochem Biophys. 1994;314:257–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

