Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis
- PMID: 9108035
- PMCID: PMC20498
- DOI: 10.1073/pnas.94.8.3668
Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis
Abstract
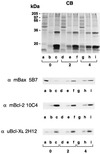
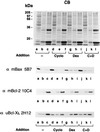
Bcl-2, Bcl-X(L), and Bax are members of the Bcl-2 family that play key roles in the regulation of apoptosis. These proteins are believed to be membrane bound and their ability to undergo both homodimerization and heterodimerization has been proposed to regulate apoptosis. Herein we report that in murine thymocytes, Bcl-2 is exclusively membrane-bound, whereas Bax is present predominantly in the cytosol and Bcl-X(L) is present in both soluble and membrane-bound forms. Induction of apoptosis in murine thymocytes by dexamethasone or gamma-irradiation shifts the subcellular locations of Bax and Bcl-X(L) from soluble to membrane-bound forms. A similar shift in the localization of Bax from the cytosol to membranes was observed in HL-60 leukemia cells upon induction of apoptosis by staurosporine. Inhibition of apoptosis with cycloheximide inhibits the movement of Bax and Bcl-X(L) in thymocytes from the cytosol into membranes induced by dexamethasone treatment. These movements may represent an important step in the pathway by which members of this family regulate apoptosis.
Figures



References
-
- Boise L H, Gottschalk A R, Quintáns J, Thompson C B. Curr Top Microbiol Immunol. 1995;200:107–121. - PubMed
-
- Reed J C. Hematol Oncol Clin North Am. 1995;9:451–473. - PubMed
-
- White E. Genes Dev. 1996;10:1–15. - PubMed
-
- Yang E, Korsmeyer S J. Blood. 1996;88:386–401. - PubMed
-
- Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

