Generation of Alzheimer beta-amyloid protein in the trans-Golgi network in the apparent absence of vesicle formation
- PMID: 9108049
- PMCID: PMC20512
- DOI: 10.1073/pnas.94.8.3748
Generation of Alzheimer beta-amyloid protein in the trans-Golgi network in the apparent absence of vesicle formation
Abstract
beta-amyloid protein (A beta) formation was reconstituted in permeabilized neuroblastoma cells expressing human Alzheimer beta-amyloid precursor protein (beta APP) harboring the Swedish double mutation associated with familial early-onset Alzheimer disease. Permeabilized cells were prepared following metabolic labeling and incubation at 20 degrees C, a temperature that allows beta APP to accumulate in the trans-Golgi network (TGN) without concomitant A beta formation. Subsequent incubation at 37 degrees C led to the generation of A beta. A beta production in the TGN persisted even under conditions in which formation of nascent post-TGN vesicles was inhibited by addition of guanosine 5'-O-(3-thiotriphosphate), a nonhydrolyzable GTP analogue, or by omission of cytosol. These and other results indicate that vesicle budding and trafficking may not be required for proteolytic metabolism of beta APP to A beta, a process that includes "gamma-secretase" cleavage within the beta APP transmembrane domain.
Figures
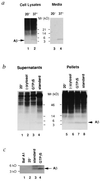
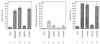


References
-
- Gandy S, Greengard P. Int Rev Neurobiol. 1994;36:29–50. - PubMed
-
- Sisodia S S, Koo E H, Beyreuther K, Unterbeck A, Price D L. Science. 1990;248:492–495. - PubMed
-
- Seubert P, Oltersdorf T, Lee M G, Barbour R, Blomquist C, Davis D L, Bryant K, Galasko D, Thal L J, Fritz L, Lieberburg I, Schenk D. Nature (London) 1993;361:260–263. - PubMed
-
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, McCormack R, Wolfert R, Selkoe D J, Lieberburg I, Schenk D. Nature (London) 1992;359:325–327. - PubMed
-
- Shoji M, Golde T E, Ghiso J, Cheung T T, Estus S, Shaffer L M, Cai X, McKay D M, Tintner R, Frangione B, Younkin S G. Science. 1992;258:126–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

