Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol
- PMID: 9108055
- PMCID: PMC20518
- DOI: 10.1073/pnas.94.8.3783
Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol
Abstract
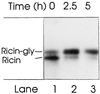
Translocation of ricin A chain to the cytosol has been proposed to take place from the endoplasmic reticulum (ER), but attempts to visualize ricin in this organelle have failed. Here we modified ricin A chain to contain a tyrosine sulfation site alone or in combination with N-glycosylation sites. When reconstituted with ricin B chain and incubated with cells in the presence of Na(2)(35)SO(4), the modified A chains were labeled. The labeling was prevented by brefeldin A and ilimaquinone, and it appears to take place in the Golgi apparatus. This method allows selective labeling of ricin molecules that have already been transported retrograde to this organelle. A chain containing C-terminal N-glycosylation sites became core glycosylated, indicating retrograde transport to the ER. In part of the toxin molecules, the A chain was released from the B chain and translocated to the cytosol. The finding that glycosylated A chain was present in the cytosol indicates that translocation takes place after transport of the toxin to the ER.
Figures



References
-
- Olsnes S, Sandvig K, Petersen O W, van Deurs B. Immunol Today. 1989;10:291–295. - PubMed
-
- Vitetta E S, Thorpe P E. Semin Cell Biol. 1991;2:47–58. - PubMed
-
- Thrush G R, Lark L R, Clinchy B C, Vitetta E S. Annu Rev Immunol. 1996;14:49–71. - PubMed
-
- Olsnes S, Pihl A. Biochemistry. 1973;12:3121–3126. - PubMed
-
- Endo Y, Mitsui K, Motizuki M, Tsurugi K. J Biol Chem. 1987;262:5908–5912. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

