Glucose transporter GLUT3: ontogeny, targeting, and role in the mouse blastocyst
- PMID: 9108057
- PMCID: PMC20520
- DOI: 10.1073/pnas.94.8.3795
Glucose transporter GLUT3: ontogeny, targeting, and role in the mouse blastocyst
Abstract
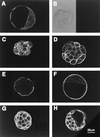
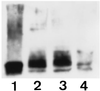
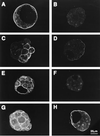
The first differentiative event in mammalian development is segregation of the inner cell mass and trophectoderm (TE) lineages. The epithelial TE cells pump fluid into the spherical blastocyst to form the blastocyst cavity. This activity is fuelled by glucose supplied through facilitative glucose transporters. However, the reported kinetic characteristics of blastocyst glucose transport are inconsistent with those of the previously identified transporters and suggested the presence of a high-affinity glucose carrier. We identified and localized the primary transporter in TE cells. It is glucose transporter GLUT3, previously described in the mouse as neuron-specific. In the differentiating embryo, GLUT3 is targeted to the apical membranes of the polarized cells of the compacted morula and then to the apical membranes of TE cells where it has access to maternal glucose. In contrast, GLUT1 was restricted to basolateral membranes of the outer TE cells in both compacted morulae and blastocysts. Using antisense oligodeoxynucleotides to specifically block protein expression, we confirmed that GLUT3 and not GLUT1 is the functional transporter for maternal glucose on the apical TE. More importantly, however, GLUT3 expression is required for blastocyst formation and hence this primary differentiation in mammalian development. This requirement is independent of its function as a glucose transporter because blastocysts will form in the absence of glucose. Thus the vectorial expression of GLUT3 into the apical membrane domains of the outer cells of the morula, which in turn form the TE cells of the blastocyst, is required for blastocyst formation.
Figures




References
-
- Fleming T P. In: Epithelial Organization and Development. 1st Ed. Fleming T P, editor. London: Chapman & Hall; 1992. pp. 111–130.
-
- Leese H J, Barton A M. J Reprod Fertil. 1984;72:9–13. - PubMed
-
- Gardner D K, Leese H J. Development (Cambridge, UK) 1988;104:423–429. - PubMed
-
- Manezwala F M, Cragoe E J, Jr, Schultz R M. Dev Biol. 1989;133:210–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

