Antigen receptor engagement delivers a stop signal to migrating T lymphocytes
- PMID: 9108078
- PMCID: PMC20541
- DOI: 10.1073/pnas.94.8.3909
Antigen receptor engagement delivers a stop signal to migrating T lymphocytes
Abstract
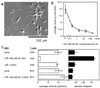
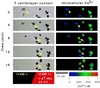
We investigated the role of the T cell antigen receptor (TcR) in control of T cell migration in an in vitro system. We used T cells from transgenic mice bearing a TcR for the lysozyme peptide 48-62 bound to I-A(k) (3A9). T cells from the 3A9 TcR transgenic mice crawled on purified intercellular adhesion molecule-1 substrates, but strikingly, stopped upon interaction with the physiological ligand, i.e., the mouse I-A(k) with covalently attached hen egg white lysozyme peptide residues 48-62 complex. TcR-triggered stopping was reversible by treatment with adhesion-strengthening phorbol esters. The microtubule organizing center of stopped cells was positioned adjacent to the site of stable cell anchorage. Direct conversion of lymphocyte function associated-1 to the high-affinity conformation with antibodies also stopped T cells in a similar manner to antigen. Thus, physiological TcR engagement triggers a stop signal through lymphocyte function associated-1. We propose that the stop signal is an early and essential event in T cell activation that also will play an important role in control of T cell migration.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

