Replication synchrony-PCR: a sampling-time-independent assay for replication synchrony in human tissues and tumors in situ
- PMID: 9108102
- PMCID: PMC20565
- DOI: 10.1073/pnas.94.8.4045
Replication synchrony-PCR: a sampling-time-independent assay for replication synchrony in human tissues and tumors in situ
Abstract
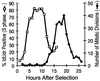
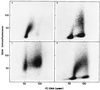
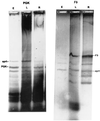
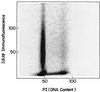
Replication synchrony within a cell population can be demonstrated by pulse-labeling followed by PCR amplification of immunoprecipitated 5-iodo-2'-deoxyuridine (IdUrd)-labeled DNA from cells of otherwise indeterminant kinetic stages. This replication synchrony-PCR approach may be valuable in understanding the dynamics of human normal tissue or solid tumor replication in situ where access for repeated sampling is severely limited. IdUrd labeling provides a sampling-time-independent method for assessing the replicative status of a cell population at the time when the label was presented. Using genes whose time of replication in S phase is already known, the presence of a cell in early or late S phase can be determined and a qualitative measure made of replication synchrony in the population. This approach was evaluated in synchronous and random cultures of Ej cells using the early replicating PGK-1 gene to identify cells in early S phase at the time of labeling and the late replicating factor IX gene to identify cells that were in late S phase. To test the feasibility of clinical application of this technique, human tumor cells from patients with advanced cancers, given IdUrd therapeutically at specified times of the day, were evaluated. In some patients, replication synchrony-PCR provided evidence of parasynchronous DNA replication in tumor cells. This technique could be appended to existing clinical studies in which BrdUrd or IdUrd is being given to patients either diagnostically or therapeutically.
Figures
Similar articles
-
DNA double labelling with IdUrd and CldUrd for spatial and temporal analysis of cell proliferation and DNA replication.Histochem J. 1992 May;24(5):251-9. doi: 10.1007/BF01046839. Histochem J. 1992. PMID: 1376726
-
A new immunocytochemical technique for ultrastructural analysis of DNA replication in proliferating cells after application of two halogenated deoxyuridines.J Histochem Cytochem. 1998 Oct;46(10):1203-9. doi: 10.1177/002215549804601014. J Histochem Cytochem. 1998. PMID: 9742078
-
Determination of DNA replication kinetics in synchronized human cells using a PCR-based assay.Nucleic Acids Res. 1993 Jul 11;21(14):3227-32. doi: 10.1093/nar/21.14.3227. Nucleic Acids Res. 1993. PMID: 8341597 Free PMC article.
-
The potential superiority of bromodeoxyuridine to iododeoxyuridine as a radiation sensitizer in the treatment of colorectal cancer.Cancer Res. 1992 Jul 1;52(13):3698-704. Cancer Res. 1992. PMID: 1617642
-
Utilization of polymerase chain reaction technology in the detection of solid tumors.Cancer. 1998 Apr 15;82(8):1419-42. doi: 10.1002/(sici)1097-0142(19980415)82:8<1419::aid-cncr1>3.0.co;2-4. Cancer. 1998. PMID: 9554517 Review.
References
-
- Smaaland R, Laerum O D, Lote K, Sletvold O, Sothern R B, Bjerknes R. Blood. 1991;77:2603–2611. - PubMed
-
- Scheving L E, Haus E, Kühl J F W, Pauly J E, Halberg F, Cardoso S. Cancer Res. 1976;36:1133–1137. - PubMed
-
- Clausen O P F, Kirkhus B, Pedersen S, Bolund L. Cell Tissue Kinet. 1985;18:445–455. - PubMed
-
- Scheving L E, Tsai T H, Scheving L A, Feuers R J. Ann NY Acad Sci. 1991;618:182–227. - PubMed
-
- Buchi K N, Hrushesky W J M, Sothern R B, Rubin N H, Moore J G. Gastroenterology. 1991;101:410–415. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

