Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments
- PMID: 9108114
- PMCID: PMC20577
- DOI: 10.1073/pnas.94.8.4113
Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments
Abstract
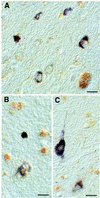
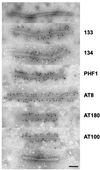
Neurofibrillary lesions made of hyperphosphorylated microtubule-associated protein tau constitute not only one of the defining neuropathological features of Alzheimer disease but also are present in a number of other neurodegenerative diseases with dementia. Here we describe a novel autosomal dominant disease named familial "multiple system tauopathy with presenile dementia," which is characterized by abundant fibrillary deposits of tau protein in both neurons and glial cells. There are no detectable deposits of beta-amyloid. The tau deposits are in the form of twisted filaments that differ in diameter and periodicity from the paired helical filaments of Alzheimer disease. They are stained by both phosphorylation-independent and -dependent anti-tau antibodies. Moreover, tau immunoreactivity coexists with heparan sulfate in affected nerve and glial cells. Tau protein extracted from filaments of familial multiple system tauopathy with presenile dementia shows a minor 72-kDa band and two major bands of 64 and 68 kDa that contain mainly hyperphosphorylated four-repeat tau isoforms of 383 and 412 amino acids.
Figures
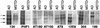

Similar articles
-
Comparison of the neurofibrillary pathology in Alzheimer's disease and familial presenile dementia with tangles.Acta Neuropathol. 1996 Jul;92(1):42-8. doi: 10.1007/s004010050487. Acta Neuropathol. 1996. PMID: 8811124
-
Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer's disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration.Acta Neuropathol. 2002 Dec;104(6):583-91. doi: 10.1007/s00401-002-0587-8. Epub 2002 Jul 13. Acta Neuropathol. 2002. PMID: 12410379
-
A mutation at codon 279 (N279K) in exon 10 of the Tau gene causes a tauopathy with dementia and supranuclear palsy.Acta Neuropathol. 1999 Jul;98(1):62-77. doi: 10.1007/s004010051052. Acta Neuropathol. 1999. PMID: 10412802
-
Ubiquitination and abnormal phosphorylation of paired helical filaments in Alzheimer's disease.Mol Neurobiol. 1991;5(2-4):399-410. doi: 10.1007/BF02935561. Mol Neurobiol. 1991. PMID: 1726645 Review.
-
Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles.J Neural Transm Suppl. 1998;53:169-80. doi: 10.1007/978-3-7091-6467-9_15. J Neural Transm Suppl. 1998. PMID: 9700655 Review.
Cited by
-
Cerebral hypometabolism and grey matter density in MAPT intron 10 +3 mutation carriers.Am J Neurodegener Dis. 2014 Dec 5;3(3):103-14. eCollection 2014. Am J Neurodegener Dis. 2014. PMID: 25628962 Free PMC article.
-
A network of RNA and protein interactions in Fronto Temporal Dementia.Front Mol Neurosci. 2015 Mar 19;8:9. doi: 10.3389/fnmol.2015.00009. eCollection 2015. Front Mol Neurosci. 2015. PMID: 25852467 Free PMC article. Review.
-
Comprehensive mRNA expression profiling distinguishes tauopathies and identifies shared molecular pathways.PLoS One. 2009 Aug 28;4(8):e6826. doi: 10.1371/journal.pone.0006826. PLoS One. 2009. PMID: 19714246 Free PMC article.
-
Atrophy patterns in IVS10+16, IVS10+3, N279K, S305N, P301L, and V337M MAPT mutations.Neurology. 2009 Sep 29;73(13):1058-65. doi: 10.1212/WNL.0b013e3181b9c8b9. Neurology. 2009. PMID: 19786698 Free PMC article.
-
Changes in proteome solubility indicate widespread proteostatic disruption in mouse models of neurodegenerative disease.Acta Neuropathol. 2018 Dec;136(6):919-938. doi: 10.1007/s00401-018-1895-y. Epub 2018 Aug 23. Acta Neuropathol. 2018. PMID: 30140941 Free PMC article.
References
-
- Goedert M, Trojanowski J Q, Lee V M-Y. In: The Molecular and Genetic Basis of Neurological Disease. 2nd Ed. Rosenberg R N, Prusiner S B, Di Mauro S, Barchi R L, editors. Boston: Butterworth–Heineman; 1997. pp. 613–622.
-
- Flament S, Delacourte A, Hémon B, Défossez A. J Neurol Sci. 1989;92:133–141. - PubMed
-
- Lee V M-Y, Balin B J, Otvos L, Trojanowski J Q. Science. 1991;251:675–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

