Expression of a borage desaturase cDNA containing an N-terminal cytochrome b5 domain results in the accumulation of high levels of delta6-desaturated fatty acids in transgenic tobacco
- PMID: 9108131
- PMCID: PMC20606
- DOI: 10.1073/pnas.94.8.4211
Expression of a borage desaturase cDNA containing an N-terminal cytochrome b5 domain results in the accumulation of high levels of delta6-desaturated fatty acids in transgenic tobacco
Abstract
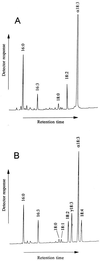
gamma-Linolenic acid (GLA; C18:3 delta(6,9,12)) is a component of the seed oils of evening primrose (Oenothera spp.), borage (Borago officinalis L.), and some other plants. It is widely used as a dietary supplement and for treatment of various medical conditions. GLA is synthesized by a delta6-fatty acid desaturase using linoleic acid (C18:2 delta(9,12)) as a substrate. To enable the production of GLA in conventional oilseeds, we have isolated a cDNA encoding the delta6-fatty acid desaturase from developing seeds of borage and confirmed its function by expression in transgenic tobacco plants. Analysis of leaf lipids from a transformed plant demonstrated the accumulation of GLA and octadecatetraenoic acid (C18:4 delta(6,9,12,15)) to levels of 13.2% and 9.6% of the total fatty acids, respectively. The borage delta6-fatty acid desaturase differs from other desaturase enzymes, characterized from higher plants previously, by the presence of an N-terminal domain related to cytochrome b5.
Figures



References
-
- Horrobin D F. Prog Lipid Res. 1992;31:163–194. - PubMed
-
- Horrobin D F. Rev Contemp Pharmacother. 1990;1:1–45.
-
- Gunstone F D. Prog Lipid Res. 1992;31:145–161. - PubMed
-
- Fieldsend A F. Biologist. 1995;42:203–207.
-
- Heinz E. In: Lipid Metabolism in Plants. Moore T S, editor. Boca Raton, FL: CRC; 1993. pp. 34–89.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

