Synergism between hypersensitive sites confers long-range gene activation by the beta-globin locus control region
- PMID: 9114030
- PMCID: PMC20763
- DOI: 10.1073/pnas.94.9.4566
Synergism between hypersensitive sites confers long-range gene activation by the beta-globin locus control region
Abstract
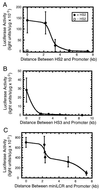
The human beta-globin locus control region (LCR) consists of four erythroid-specific DNaseI hypersensitive sites (HSs) at the 5' end of the beta-globin cluster. The LCR functions over a long distance on chromosome 11 to regulate transcription and replication of the beta-globin genes. To determine whether the HSs function independently or as an integrated unit, we analyzed the requirements for long-range transcriptional activation. If the HSs function independently, individual HSs would be expected to have long-range activity. In contrast, if long-range activity requires multiple HSs, individual HSs would have a limited functional distance. HS2, HS3, and a miniLCR containing multiple HSs, were separated from a gamma-globin promoter by fragments of phage lambda DNA. After stable transfection into K562 cells, HS2 had strong enhancer activity, but only when positioned close to the promoter. HS3 also had strong enhancer activity, although it was weaker than HS2 and more sensitive to the spacer DNA. The miniLCR had the strongest enhancer activity and functioned even at a distance of 7.3 kb. A model is proposed in which synergistic interactions between HSs confer long-range activation by creating a stable LCR nucleoprotein structure, which is competent for recruiting chromatin-modifying enzymes. These enzymes would mediate the well-characterized activity of the LCR to modulate chromatin structure.
Figures



References
-
- Felsenfeld G, Bresnick E H, Chung J, Reitman M. Molecular Biology of Hemoglobin Switching. Andover, MA: Intercept; 1995.
-
- Ptashne M. Nature (London) 1986;322:697–701. - PubMed
-
- Schleif R. Annu Rev Biochem. 1992;61:199–223. - PubMed
-
- Hager G L, Archer T K, Fragoso G, Bresnick E H, Tsukagoshi Y, John S, Smith C L. Cold Spring Harbor Symp Quant Biol. 1993;58:63–71. - PubMed
-
- Archer T K, Lefebvre P, Wolford R G, Hager G L. Science. 1992;255:1573–1576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

