A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes
- PMID: 9114038
- PMCID: PMC20771
- DOI: 10.1073/pnas.94.9.4610
A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes
Abstract
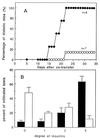
Mucosally induced immunological tolerance is an attractive strategy for preventing or treating illnesses resulting from untoward inflammatory immune reactions against self- or non-self-antigens. Oral administration of relevant autoantigens and allergens has been reported to delay or suppress onset of clinical disease in a number of experimental autoimmune and allergic disorders. However, the approach often requires repeated feeding of large amounts of tolerogens over long periods and is only partly effective in animals already systemically sensitized to the ingested antigen such as in animals already harboring autoreactive T cells, and thus presumably also in humans with an autoimmune disease. We have recently shown that oral administration of microgram amounts of antigen coupled to cholera toxin B subunit (CTB), can effectively suppress systemic T cell reactivity in naive as well as in immune animals. We now report that feeding small amounts (2-20 microg) of human insulin conjugated to CTB can effectively suppress beta cell destruction and clinical diabetes in adult nonobese diabetic (NOD) mice. The protective effect could be transferred by T cells from CTB-insulin-treated animals and was associated with reduced lesions of insulitis. Furthermore, adoptive co-transfer experiments involving injection of Thy-1,2 recipients with diabetogenic T cells from syngeneic mice and T cells from congenic Thy-1,1 mice fed with CTB-insulin demonstrated a selective recruitment of Thy-1,1 donor cells in the peripancreatic lymph nodes concomitant with reduced islet cell infiltration. These results suggest that protection against autoimmune diabetes can be achieved by feeding minute amounts of a pancreas islet cell autoantigen linked to CTB and appears to involve the selective migration and retention of protective T cells into lymphoid tissues draining the site of organ injury.
Figures

References
-
- Wells H G. J Infect Dis. 1911;8:147–171.
-
- Chase M W. Proc Soc Exp Biol. 1946;61:257–259. - PubMed
-
- Wortmann F. Allergol Immunopathol. 1977;5:15–26. - PubMed
-
- Rebien W, Puttonen E, Maasch H J, Stix E, Wahn U. Eur J Pediatr. 1982;138:341–344. - PubMed
-
- Holt P G, McMenamin C. Clin Exp Allergy. 1989;19:255–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

