LARD: a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing
- PMID: 9114039
- PMCID: PMC20772
- DOI: 10.1073/pnas.94.9.4615
LARD: a new lymphoid-specific death domain containing receptor regulated by alternative pre-mRNA splicing
Abstract
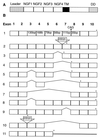
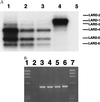
Fas and TNF-R1 are cysteine-rich cell surface receptors related to the low-affinity nerve growth factor receptor family. Engagement of these receptors by their respective ligands, FasL and tumor necrosis factor, leads to apoptosis that is signaled through a conserved intracellular portion of the receptor termed the "death domain." We have cloned a new member of this family, lymphocyte-associated receptor of death (LARD), which leads to spontaneous apoptosis when expressed in 293T cells. The expression of LARD is more tightly regulated than that of either Fas or TNF-R1 as it is found predominantly on lymphocytes (T and B cells) but not on macrophages or a number of transformed lymphocyte cell lines. Alternative pre-mRNA splicing generates at least 11 distinct isoforms of LARD. The full-length isoform, LARD-1, extends to include the transmembrane and death domains, whereas the other isoforms encode potentially secreted molecules. Naive B and T cells express very little LARD-1 but express combinations of the other isoforms. Upon T cell activation, a programmed change in alternative splicing occurs so that the full-length, membrane-bound LARD-1 predominates. This may have implications for the control of lymphocyte proliferation following activation.
Figures

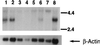


References
-
- Raff M C, Barres B A, Burne J F, Coles H S, Ishizaki Y, Jacobson M D. Science. 1993;262:695–700. - PubMed
-
- Nagata S, Golstein P. Science. 1995;267:1449–1456. - PubMed
-
- Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A. Cell. 1996;87:845–855. - PubMed
-
- Wiley R R, Schooley K, Smolak P J, Din W S, Huang C-P, Nicholl J K, Sutherland G R, Smith T D, Rauch C, Smith C S, Goodwin R G. Immunity. 1995;3:673–682. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

