A proline-rich motif in p53 is required for transactivation-independent growth arrest as induced by Gas1
- PMID: 9114050
- PMCID: PMC20783
- DOI: 10.1073/pnas.94.9.4675
A proline-rich motif in p53 is required for transactivation-independent growth arrest as induced by Gas1
Abstract
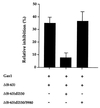
The involvement of p53 in regulating diverse cellular processes dictates that it must respond to multiple signaling mechanisms, thus coordinating the response to various "stress conditions." Genotoxic stress has served as a paradigm to dissect the transactivation-dependent branch of the pathway by which p53 can induce growth arrest. Alternate mechanisms have been invoked to explain transactivation-independent effects of p53, especially in the context of apoptosis. We have identified a p53-dependent pathway initiated by the gas1 product, a plasma membrane protein highly expressed during G0, which activates a transactivation-independent p53 growth arrest function. Through a detailed deletional analysis and site-specific mutagenesis of p53 we show that the Gas1-dependent signal transduction relies on a proline-rich region (amino acids 63-85) of murine p53. In vivo competition experiments using combinations of such mutants implicate this functional domain of p53 as a docking site in the transmission of antiproliferative signals.
Figures
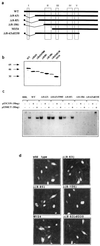
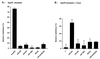

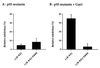
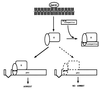
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

