Insulin secretion is regulated by the glucose-dependent production of islet beta cell macrophage migration inhibitory factor
- PMID: 9114069
- PMCID: PMC20802
- DOI: 10.1073/pnas.94.9.4782
Insulin secretion is regulated by the glucose-dependent production of islet beta cell macrophage migration inhibitory factor
Abstract
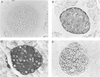
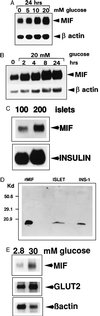
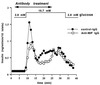
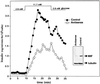
Macrophage migration inhibitory factor (MIF), originally identified as a cytokine secreted by T lymphocytes, was found recently to be both a pituitary hormone and a mediator released by immune cells in response to glucocorticoid stimulation. We report here that the insulin-secreting beta cell of the islets of Langerhans expresses MIF and that its production is regulated by glucose in a time- and concentration-dependent manner. MIF and insulin colocalize by immunocytochemistry within the secretory granules of the pancreatic islet beta cells, and once released, MIF appears to regulate insulin release in an autocrine fashion. In perifusion studies performed with isolated rat islets, immunoneutralization of MIF reduced the first and second phase of the glucose-induced insulin secretion response by 39% and 31%, respectively. Conversely, exogenously added recombinant MIF was found to potentiate insulin release. Constitutive expression of MIF antisense RNA in the insulin-secreting INS-1 cell line inhibited MIF protein synthesis and decreased significantly glucose-induced insulin release. MIF is therefore a glucose-dependent, islet cell product that regulates insulin secretion in a positive manner and may play an important role in carbohydrate metabolism.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

