C-C chemokines released by lipopolysaccharide (LPS)-stimulated human macrophages suppress HIV-1 infection in both macrophages and T cells
- PMID: 9120386
- PMCID: PMC2196157
- DOI: 10.1084/jem.185.5.805
C-C chemokines released by lipopolysaccharide (LPS)-stimulated human macrophages suppress HIV-1 infection in both macrophages and T cells
Abstract
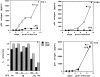
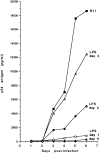
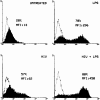
Human immunodeficiency virus-1 (HIV-1) expression in monocyte-derived macrophages (MDM) infected in vitro is known to be inhibited by lipopolysaccharide (LPS). However, the mechanisms are incompletely understood. We show here that HIV-1 suppression is mediated by soluble factors released by MDM stimulated with physiologically significant concentrations of LPS. LPS-conditioned supernatants from MDM inhibited HIV-1 replication in both MDM and T cells. Depletion of C-C chemokines (RANTES, MIP-1 alpha, and MIP-1 beta) neutralized the ability of LPS-conditioned supernatants to inhibit HIV-1 replication in MDM. A combination of recombinant C-C chemokines blocked HIV-1 infection as effectively as LPS. Here, we report an inhibitory effect of C-C chemokines on HIV replication in primary macrophages. Our results raise the possibility that monocytes may play a dual role in HIV infection: while representing a reservoir for the virus, they may contribute to the containment of the infection by releasing factors that suppress HIV replication not only in monocytes but also in T lymphocytes.
Figures




References
-
- Stoler MH, Eskin TA, Benn S, Angerer RC, Angerer LM. Human T-cell lymphotrophic virus type III infection of the central nervous system. Preliminary in situ analysis. JAMA. 1986;256:2360–2364. - PubMed
-
- Koenig S, Gendelman HE, Orenstein JM, Canto MCD, Pezeshkpour GM, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science (Wash DC) 1986;233:1089–1093. - PubMed
-
- Weiss SH, Goedert JJ, Gartner S, Popovic M, Waters D, Markham P, Di Marzo F, Veronese, Gail MH, Barkley WE, Gibbons J, et al. Risk of human immunodeficiency virus infection among laboratory workers. Science (Wash DC) 1988;239:68–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

