Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells
- PMID: 9120387
- PMCID: PMC2196166
- DOI: 10.1084/jem.185.5.817
Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells
Abstract
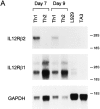
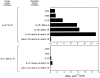
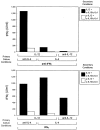
The developmental commitment to a T helper 1 (Th1)- or Th2-type response can significantly influence host immunity to pathogens. Extinction of the IL-12 signaling pathway during early Th2 development provides a mechanism that allows stable phenotype commitment. In this report we demonstrate that extinction of IL-12 signaling in early Th2 cells results from a selective loss of IL-12 receptor (IL-12R) beta 2 subunit expression. To determine the basis for this selective loss, we examined IL-12R beta 2 subunit expression during Th cell development in response to T cell treatment with different cytokines. IL-12R beta 2 is not expressed by naive resting CD4+ T cells, but is induced upon antigen activation through the T cell receptor. Importantly, IL-4 and IFN-gamma were found to significantly modify IL-12 receptor beta 2 expression after T cell activation. IL-4 inhibited IL-12R beta 2 expression leading to the loss of IL-12 signaling, providing an important point of regulation to promote commitment to the Th2 pathway. IFN-gamma treatment of early developing Th2 cells maintained IL-12R beta 2 expression and restored the ability of these cells to functionally respond to IL-12, but did not directly inhibit IL-4 or induce IFN-gamma production. Thus, IFN-gamma may prevent early Th cells from premature commitment to the Th2 pathway. Controlling the expression of the IL-12R beta 2 subunit could be an important therapeutic target for the redirection of ongoing Th cell responses.
Figures




References
-
- Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. - PubMed
-
- Sher A, Coffman RL. Regulation of immunity to parasites by T cells and T cell-derived lymphokines. Ann Rev Immunol. 1992;10:385–409. - PubMed
-
- Seder RA, Paul WE. Acquisition of lymphokineproducing phenotype by CD4+T cells. Ann Rev Immunol. 1994;12:635–673. - PubMed
-
- Hsieh C-S, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science (Wash DC) 1993;260:547–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

