Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization
- PMID: 9120391
- PMCID: PMC2196167
- DOI: 10.1084/jem.185.5.855
Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization
Abstract
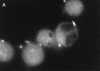
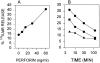
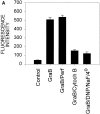
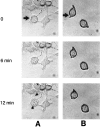
Granzyme B (GraB) induces apoptosis in the presence of perforin. Perforin polymerizes in the cell membrane to form a nonspecific ion pore, but it is not known where GraB acts to initiate the events that ultimately lead to apoptosis. It has been hypothesized that GraB enters the target cell through a perforin channel and then initiates apoptosis by cleaving and activating members of the ICE/Ced-3 family of cell death proteases. To determine if GraB can enter the cell, we treated YAC-1 or HeLa cells with FITC-labeled GraB and measured intracellular fluorescence with a high sensitivity CCD camera and image analyzer. GraB was internalized and found diffusely dispersed in the cell cytoplasm within 10 min. Uptake was inhibited at low temperature (4 degrees C) and by pretreatment with metabolic inhibitors, NaF and DNP, or cytochalasin B, a drug that both blocks microfilament formation, and FITC-GraB remained on the cell membrane localized in patches. With the simultaneous addition of perforin and FITC-GraB, no significant increase in cytoplasmic fluorescence was observed over that found in cells treated only with FITC-GraB. However, FITC-GraB was now detected in the nucleus of apoptotic cells labeling apoptotic bodies and localized areas within and along the nuclear membrane. The ability of GraB to enter cells in the absence of perforin was reexamined using anti-GraB antibody immunogold staining of ultrathin cryosections of cells incubated with GraB. Within 15 min, gold particles were detected both on the plasma membrane and in the cytoplasm of cells with some gold staining adjacent to the nuclear envelope but not in the nucleus. Cells internalizing GraB in the absence of perforin appeared morphologically normal by Hoechst staining and electron microscopy. GraB directly microinjected into the cytoplasm of B16 melanoma cells induced transient plasma membrane blebbing and nuclear coarsening but the cells did not become frankly apoptotic unless perforin was added. We conclude that GraB can enter cells autonomously but that perforin initiates the apoptotic process and the entry of GraB into the nucleus.
Figures

















References
-
- Kaegi D, Vignaux F, Ledermann B, Buerki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science (Wash DC) 1994;265:528–530. - PubMed
-
- Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature (Lond) 1994;370:650–652. - PubMed
-
- Kaegi D, Ledermann B, Buerki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature (Lond) 1994;369:31–37. - PubMed
-
- Kojima H, Shinohara N, Hanaoka S, Someya Shirota Y, Takagaki Y, Ohno H, Saito T, Katayama T, Yagita H, Okumura K, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

