A T helper cell 2 (Th2) immune response against non-self antigens modifies the cytokine profile of autoimmune T cells and protects against experimental allergic encephalomyelitis
- PMID: 9120396
- PMCID: PMC2196156
- DOI: 10.1084/jem.185.5.901
A T helper cell 2 (Th2) immune response against non-self antigens modifies the cytokine profile of autoimmune T cells and protects against experimental allergic encephalomyelitis
Abstract
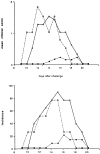
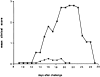
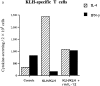
Experimental allergic encephalomyelitis (EAE) is an autoimmune disease of the central nervous system (CNS), and the most commonly used experimental model for multiple sclerosis. It is mediated by autoreactive T cell clones exhibiting a T helper cell (Th) 1 cytokine profile. Nonencephalitogenic T lymphocytes specific for self or exogenous antigens have been found to suppress encephalitogenic T cell responses and to protect against autoimmune disease. The mechanisms by which exogenous antigens modulate autoimmunity are not fully understood. In this study, we tested the hypothesis that a Th2-type immune response against an exogenous, nonself antigen, keyhole limpet hemocyanin (KLH), by releasing IL-4 in the microenvironment, could shift the cytokine profile of encephalitogenic T cells from an inflammatory Th1 to a protective Th2 type. SJL/J mice were preimmunized with the KLH in incomplete Freund's adjuvant to induce a population of Th2 memory cells that would be expected to release Th2 cytokines when activated by the specific antigen at the time of EAE induction. Four weeks later, mice received an encephalitogenic challenge containing guinea pig myelin in complete Freund's adjuvant with or without KLH. All KLH primed animals not receiving the exogenous antigen at the time of EAE induction developed a severe clinical disease indistinguishable from control mice not KLH primed. In contrast, animals preimmunized and challenged with the encephalitogenic inoculum containing KLH showed either no, or markedly reduced, clinical signs. Enzyme-linked immunospot analysis demonstrated that KLH-specific T cells in the primed mice were producing IL-4 characteristic of Th2 cells. In the KLH-primed and restimulated mice, the cytokine profile of the autoreactive, myelin basic protein-specific T cells was shifted from an inflammatory Th1 towards a protective Th2 type. We infer that the presence of IL-4 secreted by KLH-specific memory Th2 cells in the lymphoid system microenvironment in which the autoreactive T cells were engaged by the encephalitogenic stimulus were able to bias their cytokine profile towards a protective Th2 phenotype. This interpretation is supported by the observation that the protective effect of preimmunization with KLH was overcome by rm-IL-12, which inhibited the production of IL-4 by the Th1 cells and biased the autoimmune response to a predominantly Th1 type. Since IL-4 mRNA could not be detected by reverse transcriptase PCR in the CNS, the protective effect was inferred to be mediated by Th2 cells in the lymphoid system, and not the target organ. We conclude that exogenous, nonself antigens that can induce Th2 responses, can modify the cytokine environment sufficiently to alter the cytokine phenotype of inflammatory, autoreactive T cell clones, and ultimately, to provide significant protection against EAE and possibly other T cell-mediated autoimmune diseases.
Figures

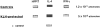
References
-
- Ben-Nun A, Wekerle H, Cohen IR. The rapid isolation of clonable antigen-specific T lymphocytes lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981;11:195–199. - PubMed
-
- Pettinelli CB, Fritz RB, Chau CHJ, McFarlin DE. Encephalitogenic activity of guinea pig myelin basic protein in the SJL/J mouse. J Immunol. 1982;129:1209–1211. - PubMed
-
- Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;1:34–38. - PubMed
-
- Miller SD, Karpus WJ. The immunopathogenesis and regulation of T-cell–mediated demyelinating diseases. Immunol Today. 1994;8:356–361. - PubMed
-
- Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science (Wash DC) 1994;265:1237–1240. - PubMed

