Regulation of E2F through ubiquitin-proteasome-dependent degradation: stabilization by the pRB tumor suppressor protein
- PMID: 9122175
- PMCID: PMC20068
- DOI: 10.1073/pnas.94.6.2221
Regulation of E2F through ubiquitin-proteasome-dependent degradation: stabilization by the pRB tumor suppressor protein
Abstract
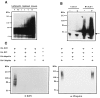
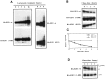
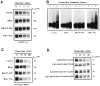
The E2F family of transcription factors plays a key role in regulating cell-cycle progression. Accordingly, E2F is itself tightly controlled by a series of transcriptional and posttranscriptional events. Here we provide evidence that E2FI protein levels are regulated by the ubiquitin-proteasome-dependent degradation pathway. An analysis of E2F1 mutants identified a conserved carboxyl-terminal region, which is required for eliciting ubiquitination and protein turnover. Fusion of this E2F1 carboxyl-terminal sequence to a heterologous protein, GAL4, resulted in destabilization of GAL4. Previous studies identified an overlapping region of E2F1 that facilitates complex formation with retinoblastoma tumor suppressor protein, pRB, and we found that pRB blocks ubiquitination and stabilizes E2F1. These results suggest a new mechanism for controlling the cell-cycle regulatory activity of E2F1.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

