Stat5 is a physiological substrate of the insulin receptor
- PMID: 9122188
- PMCID: PMC20081
- DOI: 10.1073/pnas.94.6.2295
Stat5 is a physiological substrate of the insulin receptor
Abstract
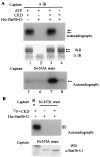
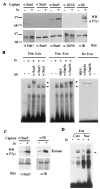
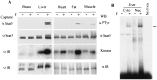
Using the cytoplasmic domain of the insulin receptor (IR) in a yeast two-hybrid screen, we identified a cDNA clone encoding the C-terminal 308 amino acids of human Stat5b (Stat5b-Ct). Stat5b-Ct is tyrosine phosphorylated by purified IR kinase domain in vitro. Insulin stimulates tyrosine phosphorylation of overexpressed Stat5b-Ct and endogenous Stat5 in cells overexpressing IR. Stat5 may be a direct target of the IR and, as a member of the Stat family of transcription factors, may play a role in the regulation of gene transcription by insulin. In support of this hypothesis, perfusion of mouse liver with insulin promotes rapid tyrosine phosphorylation of Stat5 and activation of Stat5 DNA binding. Moreover, refeeding of fasted mice leads to rapid tyrosine phosphorylation and stimulation of enhanced DNA-binding activity of Stat5 extracted from liver, skeletal muscle, and adipose tissues. Taken together, our data strongly suggest that IR interacts with and phosphorylates Stat5 in vitro and in tissues physiologically sensitive to insulin.
Figures


Similar articles
-
Dual mechanism of signal transducer and activator of transcription 5 activation by the insulin receptor.Mol Endocrinol. 2002 Dec;16(12):2764-79. doi: 10.1210/me.2002-0017. Mol Endocrinol. 2002. PMID: 12456798
-
Growth hormone-induced tyrosyl phosphorylation and deoxyribonucleic acid binding activity of Stat5A and Stat5B.Endocrinology. 1997 Aug;138(8):3426-34. doi: 10.1210/endo.138.8.5332. Endocrinology. 1997. PMID: 9231797
-
Insulin Induction of SOCS-2 and SOCS-3 mRNA expression in C2C12 Skeletal Muscle Cells Is Mediated by Stat5*.J Biol Chem. 2001 Jun 8;276(23):20703-10. doi: 10.1074/jbc.M101014200. Epub 2001 Mar 13. J Biol Chem. 2001. PMID: 11279166
-
A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b.J Biol Chem. 2000 Dec 15;275(50):39718-26. doi: 10.1074/jbc.M005615200. J Biol Chem. 2000. Retraction in: J Biol Chem. 2011 Mar 25;286(12):10888. doi: 10.1074/jbc.a110.005615. PMID: 10993888 Retracted.
-
Growth hormone pulse-activated STAT5 signalling: a unique regulatory mechanism governing sexual dimorphism of liver gene expression.Novartis Found Symp. 2000;227:61-74; discussion 75-81. doi: 10.1002/0470870796.ch5. Novartis Found Symp. 2000. PMID: 10752065 Review.
Cited by
-
Skeletal muscle mitochondrial function and exercise capacity are not impaired in mice with knockout of STAT3.J Appl Physiol (1985). 2019 Oct 1;127(4):1117-1127. doi: 10.1152/japplphysiol.00003.2019. Epub 2019 Sep 12. J Appl Physiol (1985). 2019. PMID: 31513449 Free PMC article.
-
An extracellular receptor tyrosine kinase motif orchestrating intracellular STAT activation.Nat Commun. 2022 Nov 14;13(1):6953. doi: 10.1038/s41467-022-34539-4. Nat Commun. 2022. PMID: 36376313 Free PMC article.
-
Leptin signal transduction in the HP75 human pituitary cell line.Pituitary. 2000 Dec;3(4):211-20. doi: 10.1023/a:1012994712851. Pituitary. 2000. PMID: 11788008
-
Effects of heterologous kinase domains on growth factor receptor specificity.Cell Signal. 2024 Oct;122:111307. doi: 10.1016/j.cellsig.2024.111307. Epub 2024 Jul 22. Cell Signal. 2024. PMID: 39048037
-
Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses.Proc Natl Acad Sci U S A. 2011 Mar 15;108(11):4340-5. doi: 10.1073/pnas.1011115108. Epub 2011 Feb 23. Proc Natl Acad Sci U S A. 2011. PMID: 21368206 Free PMC article.
References
-
- Draznin B, Melmed S, Leroith D, editors. Insulin Action, Molecular and Cellular Biology of Diabetes Mellitus. II. New York: Liss; 1989.
-
- Ebina Y, Ellis L, Jarnagin K, Edery M, Graf L, Clauster E, Ou J H, Masiarz F, Kan Y W, Goldfine I D, Roth R A, Rutter W J. Cell. 1989;40:747–758. - PubMed
-
- Chou C K, Dull T J, Russel D S, Gherzi R, Lebwohl D, Ullrich A, Rosen O M. J Biol Chem. 1987;262:1842–1847. - PubMed
-
- Cheatham B, Kahn C R. Endocrine Review. 1995;16:117–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

