Dissecting the thrombopoietin receptor: functional elements of the Mpl cytoplasmic domain
- PMID: 9122198
- PMCID: PMC20091
- DOI: 10.1073/pnas.94.6.2350
Dissecting the thrombopoietin receptor: functional elements of the Mpl cytoplasmic domain
Abstract
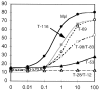
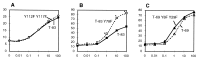
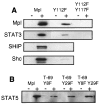
Thrombopoietin (TPO) acts through its receptor, Mpl, to stimulate the proliferation and maturation of megakaryocytes and their progenitors. The Mpl cytoplasmic domain controls this process through assembly of an active signaling complex using various receptor docking sites. In this report, eight carboxyl truncations of the 121-aa murine Mpl cytoplasmic domain were tested for the ability to support growth of a cytokine-dependent cell line (Ba/F3) and for their capacity to induce TPO-stimulated tyrosine phosphorylation of specific signaling proteins. Point mutations of the five tyrosine residues in the cytoplasmic domain of the receptor were subsequently used to confirm our conclusions. From these studies we demonstrate that: (i) TPO-induced proliferation is moderately reduced by truncation of as many as 53 C-terminal amino acids of Mpl, including the sites of receptor tyrosine phosphorylation; (ii) truncation/mutation of residues 69-83 of the Mpl cytoplasmic domain enhances proliferative signaling, perhaps mediated by a decrease in receptor-driven cellular differentiation; (iii) Mpl can be phosphorylated at either Y112 or Y117 but not at the three proximal cytoplasmic tyrosine residues (Y8, Y29, and Y78); (iv) Y112 of Mpl is necessary for tyrosine phosphorylation of Shc and Shc-associated p145 (SHIP); and (v) unlike STAT3, STAT5 is partially phosphorylated in the absence of any tyrosine residues in the Mpl cytoplasmic domain. These studies identify subdomains of Mpl necessary for activation of several critical signaling pathways and point to two potentially novel mechanisms of TPO-induced signal transduction, an indirect pathway to STAT5 activation and a differentiation domain that acts by limiting proliferation.
Figures


References
-
- Kaushansky K, Lok S, Holly R D, Broudy V C, Lin N, et al. Nature (London) 1994;369:568–571. - PubMed
-
- Broudy V C, Lin N L, Fox N, Taga T, Saito M, Kaushansky K. Blood. 1996;88:2026–2032. - PubMed
-
- Morella K K, Bruno E, Kumaki S, Lai C F, Fu J, Wang H M, Murray L, Hoffman R, Timour M, Bénit L, Gisselbrecht S, Zhvans H, Wojchowski D M, Bauman H, Gearing D P. Blood. 1995;86:557–571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

