Function and regulation of heat shock factor 2 during mouse embryogenesis
- PMID: 9122205
- PMCID: PMC20098
- DOI: 10.1073/pnas.94.6.2392
Function and regulation of heat shock factor 2 during mouse embryogenesis
Abstract
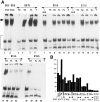
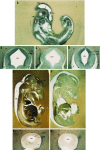
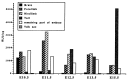
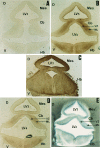
The spontaneous expression of heat shock genes during development is well documented in many animal species, but the mechanisms responsible for this developmental regulation are only poorly understood. In vertebrates, additional heat shock transcription factors, distinct from the heat shock factor 1 (HSF1) involved in the stress response, were suggested to be involved in this developmental control. In particular, the mouse HSF2 has been found to be active in testis and during preimplantation development. However, the role of HSF2 and its mechanism of activation have remained elusive due to the paucity of data on its expression during development. In this study, we have examined HSF2 expression during the postimplantation phase of mouse development. Our data show a developmental regulation of HSF2, which is expressed at least until 15.5 days of embryogenesis. It becomes restricted to the central nervous system during the second half of gestation. It is expressed in the ventricular layer of the neural tube which contains mitotically active cells but not in postmitotic neurons. Parallel results were obtained for mRNA, protein, and activity levels, demonstrating that the main level of control was transcriptional. The detailed analysis of the activity of a luciferase reporter gene under the control of the hsp70.1 promoter, as well as the description of the protein expression patterns of the major heat shock proteins in the central nervous system, show that HSF2 and heat shock protein expression domains do not coincide. This result suggests that HFS2 might be involved in other regulatory developmental pathways and paves the way to new functional approaches.
Figures

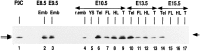
References
-
- Pauli D, Tissières A. In: Developmental Expression of the Heat Shock Genes in Drosophila melanogaster. Morimoto R I, Tissières A, Georgopoulos C, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. pp. 361–378.
-
- Sarge K D, Zimarino V, Holm K, Wu C, Morimoto R I. Genes Dev. 1991;5:1902–1911. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

