Genetically engineered superantigens as tolerable antitumor agents
- PMID: 9122222
- PMCID: PMC20115
- DOI: 10.1073/pnas.94.6.2489
Genetically engineered superantigens as tolerable antitumor agents
Abstract
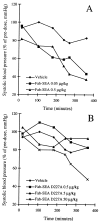
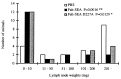
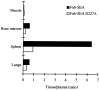
Superantigens (SAg) are a family of bacterial and viral proteins with strong immunostimulatory properties. SAg bound to major histocompatibility complex (MHC) class II molecules activate a high frequency of T cells and represent the most potent known activators of T cells to date. To explore the use of SAg for T cell-based tumor therapy we have created a tumor-reactive SAg by engineering a fusion protein composed of a tumor-reactive mAb (C215Fab) and the bacterial SAg staphylococcal enterotoxin A (SEA). A point mutation D227A was introduced at the major MHC class II binding site in SEA to reduce systemic toxicity. Treatment of tumor bearing mice with the Fab-SEA D227A fusion protein resulted in profound antitumor effects with a markedly reduced toxicity as compared with the wild-type Fab-SEA fusion protein. The reduced toxicity was probably due to a weak distribution of the SEA D227A fusion protein in tissues with a high MHC class II expression and low systemic cytokine levels as exhibited in mice and rabbits. The data presented demonstrate the efficacy of immunoconjugates containing a mutated SAg in directing a T cell attack against tumor cells with minimal systemic immune activation.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

