Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training
- PMID: 9122258
- PMCID: PMC20151
- DOI: 10.1073/pnas.94.6.2693
Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training
Abstract
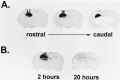
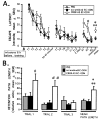
Extensive evidence suggests that long term memory (LTM) formation is dependent on the activation of neuronal second messenger systems and requires protein synthesis. The cAMP response element binding protein (CREB) is a constitutively expressed regulatory transcription factor that couples changes in second messenger levels to changes in cellular transcription. Several recent studies suggest that CREB and related transcription factors regulate gene expression necessary for neuronal plasticity and LTM. However, the role of CREB, within defined mammalian brain structures, in mediating the cellular events underlying LTM formation has not been investigated. We examined whether CREB-mediated transcription within the dorsal hippocampus is critical to LTM consolidation of water maze spatial training, which is known to depend on dorsal hippocampal function. Pretraining infusions of antisense oligodeoxynucleotides (ODN) directed against CREB mRNA were used to disrupt hippocampal CREB protein levels in adult rats. Control groups received pretraining infusions of ODN of the same base composition but in a randomized order (scrambled ODN) or buffer. Task acquisition and memory up to 4 h (i.e., short term memory) were similar in CREB antisense ODN and control groups. In contrast, CREB antisense ODN-infused rats exhibited significantly impaired memory 48 h later (i.e., LTM). Moreover, administration of antisense ODN 1 day after training did not affect subsequent retention performance. These findings provide the first evidence that CREB-mediated transcription is integral to hippocampal-dependent memory consolidation processes.
Figures


Similar articles
-
CREB antisense oligodeoxynucleotide administration into the dorsal hippocampal CA3 region impairs long- but not short-term spatial memory in mice.Learn Mem. 2006 Jul-Aug;13(4):465-72. doi: 10.1101/lm.249306. Learn Mem. 2006. PMID: 16882863 Free PMC article.
-
Lentivirus-mediated chronic expression of dominant-negative CREB in the dorsal hippocampus impairs memory for place learning and contextual fear conditioning.Neurobiol Learn Mem. 2013 Jan;99:10-6. doi: 10.1016/j.nlm.2012.10.008. Epub 2012 Oct 27. Neurobiol Learn Mem. 2013. PMID: 23110949
-
Long-term memory for place learning is facilitated by expression of cAMP response element-binding protein in the dorsal hippocampus.Learn Mem. 2007 Mar 8;14(3):195-9. doi: 10.1101/lm.395407. Print 2007 Mar. Learn Mem. 2007. PMID: 17351144
-
Molecular and cellular mechanisms of memory allocation in neuronetworks.Neurobiol Learn Mem. 2008 Mar;89(3):285-92. doi: 10.1016/j.nlm.2007.08.017. Epub 2007 Oct 24. Neurobiol Learn Mem. 2008. PMID: 17962049 Free PMC article. Review.
-
The impact of flavonoids on memory: physiological and molecular considerations.Chem Soc Rev. 2009 Apr;38(4):1152-61. doi: 10.1039/b800422f. Epub 2009 Jan 20. Chem Soc Rev. 2009. PMID: 19421586 Review.
Cited by
-
Cognitive enhancement with rosiglitazone links the hippocampal PPARγ and ERK MAPK signaling pathways.J Neurosci. 2012 Nov 21;32(47):16725-35a. doi: 10.1523/JNEUROSCI.2153-12.2012. J Neurosci. 2012. PMID: 23175826 Free PMC article.
-
Cyclic AMP response element-binding protein (CREB) transcription factor in astrocytic synaptic communication.Front Synaptic Neurosci. 2023 Jan 4;14:1059918. doi: 10.3389/fnsyn.2022.1059918. eCollection 2022. Front Synaptic Neurosci. 2023. PMID: 36685081 Free PMC article. Review.
-
Role of hippocampal signaling pathways in long-term memory formation of a nonassociative learning task in the rat.Learn Mem. 2000 Sep-Oct;7(5):333-40. doi: 10.1101/lm.34600. Learn Mem. 2000. PMID: 11040265 Free PMC article.
-
Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning.J Neurosci. 2000 Nov 1;20(21):8177-87. doi: 10.1523/JNEUROSCI.20-21-08177.2000. J Neurosci. 2000. PMID: 11050141 Free PMC article.
-
Temporal requirement of C/EBPbeta in the amygdala following reactivation but not acquisition of inhibitory avoidance.Learn Mem. 2007 Jul 18;14(7):504-11. doi: 10.1101/lm.598307. Print 2007 Jul. Learn Mem. 2007. PMID: 17644752 Free PMC article.
References
-
- Davis H, Squire L R. Psychol Bull. 1984;96:518–559. - PubMed
-
- Glassman E. Annu Rev Biochem. 1969;38:605–646. - PubMed
-
- Barondes S H. Int Rev Neurobiol. 1970;12:177–205. - PubMed
-
- Uphouse L L, MacInnes J W, Schlesinger K. Behav Genet. 1974;4:29–81. - PubMed
-
- Montminy M R, Gonzalez G A, Yamamoto K K. Trends Neurosci. 1990;13:184–188. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

