Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy
- PMID: 9126922
- PMCID: PMC2196281
- DOI: 10.1084/jem.185.8.1413
Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy
Abstract
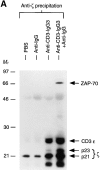
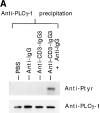
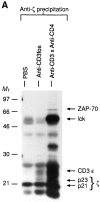
Anti-CD3 monoclonal antibodies (mAbs) are potent immunosuppressive agents used in clinical transplantation. However, the activation-related adverse side effects associated with these mAbs have prompted the development of less toxic nonmitogenic anti-CD3 mAb therapies. At present, the functional and biochemical consequences of T cell exposure to nonmitogenic anti-CD3 is unclear. In this study, we have examined the early signaling events triggered by a nonmitogenic anti-CD3 mAb. Like the mitogenic anti-CD3 mAb, nonnmitogenic anti-CD3 triggered changes in the T cell receptor (TCR) complex, including zeta chain tyrosine phosphorylation and ZAP-70 association. However, unlike the mitogenic anti-CD3 stimulation, nonmitogenic anti-CD3 was ineffective at inducing the highly phosphorylated form of zeta (p23) and tyrosine phosphorylation of the associated ZAP-70 tyrosine kinase. This proximal signaling deficiency correlated with minimal phospholipase Cgamma-1 phosphorylation and failure to mobilize detectable Ca2+. Not only did biochemical signals delivered by nonmitogenic anti-CD3 resemble altered peptide ligand signaling, but exposure of Th1 clones to nonmitogenic anti-CD3 also resulted in functional anergy. Finally, a bispecific anti-CD3 X anti-CD4 F(ab)'2 reconstituted early signal transduction events and induced proliferation, suggesting that defective association of lck with the TCR complex may underlie the observed signaling differences between the mitogenic and nonmitogenic anti-CD3.
Figures













References
-
- Ortho Multicenter Transplant Study Group. A randomized clinical trial of OKT3 monoclonal antibody for acute rejection of cadaveric renal transplants. N Engl J Med. 1985;313:337–341. - PubMed
-
- Charpentier B, Hiesse C, Lantz O, Ferran C, Stephens S, O'Shaugnessy D, Bodmer M, Benoit G, Bach JF, Chatenoud L. Evidence that antihuman tumor necrosis factor monoclonal antibody prevents OKT3-induced acute syndrome. Transplantation. 1992;54:997–1002. - PubMed
-
- Ferran C, Sheehan K, Dy M, Schreiber R, Merite S, Landais P, Noel L, Grau G, Bluestone JA, Bach JF, Chatenoud L. Cytokine related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol. 1990;20:509–515. - PubMed
-
- Kan EAR, Wright SD, Welte K, Wang CY. Fc receptors on monocytes cause OKT3-treated lymphocytes to internalize T3 and to secrete IL-2. Cell Immunol. 1986;98:181–187. - PubMed
-
- Alegre ML, Tso JY, Sattar HA, Smith J, Desalle F, Cole M, Bluestone JA. An anti-murine CD3 monoclonal antibody with a low affinity for Fc gamma receptors suppresses transplantation responses while minimizing acute toxicity and immunogenicity. J Immunol. 1995;155:1544–1555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

