Structural protein 4.1 in the nucleus of human cells: dynamic rearrangements during cell division
- PMID: 9128242
- PMCID: PMC2139783
- DOI: 10.1083/jcb.137.2.275
Structural protein 4.1 in the nucleus of human cells: dynamic rearrangements during cell division
Abstract
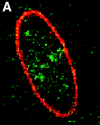
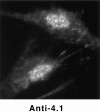
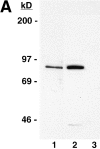
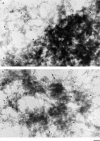
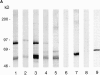
Structural protein 4.1, first identified as a crucial 80-kD protein in the mature red cell membrane skeleton, is now known to be a diverse family of protein isoforms generated by complex alternative mRNA splicing, variable usage of translation initiation sites, and posttranslational modification. Protein 4.1 epitopes are detected at multiple intracellular sites in nucleated mammalian cells. We report here investigations of protein 4.1 in the nucleus. Reconstructions of optical sections of human diploid fibroblast nuclei using antibodies specific for 80-kD red cell 4.1 and for 4.1 peptides showed 4.1 immunofluorescent signals were intranuclear and distributed throughout the volume of the nucleus. After sequential extractions of cells in situ, 4.1 epitopes were detected in nuclear matrix both by immunofluorescence light microscopy and resinless section immunoelectron microscopy. Western blot analysis of fibroblast nuclear matrix protein fractions, isolated under identical extraction conditions as those for microscopy, revealed several polypeptide bands reactive to multiple 4.1 antibodies against different domains. Epitope-tagged protein 4.1 was detected in fibroblast nuclei after transient transfections using a construct encoding red cell 80-kD 4.1 fused to an epitope tag. Endogenous protein 4.1 epitopes were detected throughout the cell cycle but underwent dynamic spatial rearrangements during cell division. Protein 4.1 was observed in nucleoplasm and centrosomes at interphase, in the mitotic spindle during mitosis, in perichromatin during telophase, as well as in the midbody during cytokinesis. These results suggest that multiple protein 4.1 isoforms may contribute significantly to nuclear architecture and ultimately to nuclear function.
Figures


















References
-
- Anderson RA, Correas I, Mazzucco C, Castle JD, Marchesi VT. Tissue-specific analogues of erythrocyte protein 4.1 retain functional domains. J Cell Biochem. 1988;37:269–284. - PubMed
-
- Bachs O, Lanini L, Serratosa J, Coll MJ, Bastos K, Alique R, Ruis E, Carafoli E. Calmodulin binding proteins in the nuclei of quiescent and proliferatively activated rat liver cells. J Biol Chem. 1990;265:18595–18599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

