Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor
- PMID: 9128254
- PMCID: PMC2139769
- DOI: 10.1083/jcb.137.2.445
Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor
Erratum in
- J Cell Biol 1997 Jun 2;137(5):1197
Abstract
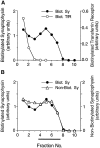
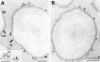
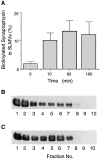
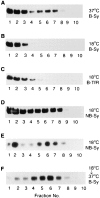
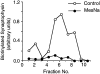
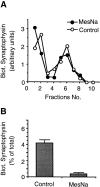
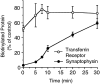
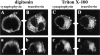
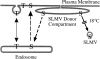
We have characterized the compartment from which synaptic-like microvesicles (SLMVs), the neuroendocrine counterpart of neuronal synaptic vesicles, originate. For this purpose we have exploited the previous observation that newly synthesized synaptophysin, a membrane marker of synaptic vesicles and SLMVs, is delivered to the latter organelles via the plasma membrane and an internal compartment. Specifically, synaptophysin was labeled by cell surface biotinylation of unstimulated PC12 cells at 18 degrees C, a condition which blocked the appearance of biotinylated synaptophysin in SLMVs and in which there appeared to be no significant exocytosis of SLMVs. The majority of synaptophysin labeled at 18 degrees C with the membrane-impermeant, cleavable sulfo-NHS-SS-biotin was still accessible to extracellularly added MesNa, a 150-D membrane-impermeant thiol-reducing agent, but not to the 68,000-D protein avidin. The SLMVs generated upon reversal of the temperature to 37 degrees C originated exclusively from the membranes containing the MesNa-accessible rather than the MesNa-protected population of synaptophysin molecules. Biogenesis of SLMVs from MesNa-accessible membranes was also observed after a short (2 min) biotinylation of synaptophysin at 37 degrees C followed by chase. In contrast to synaptophysin, transferrin receptor biotinylated at 18 degrees or 37 degrees C became rapidly inaccessible to MesNa. Immunofluorescence and immunogold electron microscopy of PC12 cells revealed, in addition to the previously described perinuclear endosome in which synaptophysin and transferrin receptor are colocalized, a sub-plasmalemmal tubulocisternal membrane system distinct from caveolin-positive caveolae that contained synaptophysin but little, if any, transferrin receptor. The latter synaptophysin was selectively visualized upon digitonin permeabilization and quantitatively extracted, despite paraformaldehyde fixation, by Triton X-100. Synaptophysin biotinylated at 18 degrees C was present in these subplasmalemmal membranes. We conclude that SLMVs originate from a novel compartment that is connected to the plasma membrane via a narrow membrane continuity and lacks transferrin receptor.
Figures

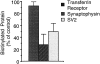



References
-
- Anderson RGW. Potocytosis of small molecules and ions by caveolae. Trends Cell Biol. 1993;3:69–72. - PubMed
-
- Bauerfeind R, Régnier-Vigouroux A, Flatmark T, Huttner WB. Selective storage of acetylcholine, but not catecholamines, in neuroendo-crine synaptic-like microvesicles off early endosomal origin. Neuron. 1993;11:105–121. - PubMed
-
- Bennett MK, Scheller RH. A molecular description of synaptic vesicle membrane trafficking. Annu Rev Biochem. 1994;63:63–100. - PubMed

