Isolated P-selectin glycoprotein ligand-1 dynamic adhesion to P- and E-selectin
- PMID: 9128259
- PMCID: PMC2139768
- DOI: 10.1083/jcb.137.2.509
Isolated P-selectin glycoprotein ligand-1 dynamic adhesion to P- and E-selectin
Abstract
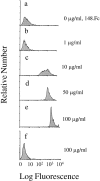
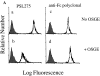
Leukocyte adhesion to vascular endothelium under flow involves an adhesion cascade consisting of multiple receptor pairs that may function in an overlapping fashion. P-selectin glycoprotein ligand-1 (PSGL-1) and L-selectin have been implicated in neutrophil adhesion to P- and E-selectin under flow conditions. To study, in isolation, the interaction of PSGL-1 with P- and E-selectin under flow, we developed an in vitro model in which various recombinant regions of extracellular PSGL-1 were coupled to 10-microm-diameter microspheres. In a parallel plate chamber with well defined flow conditions, live time video microscopy analyses revealed that microspheres coated with PSGL-1 attached and rolled on 4-h tumor necrosis factor-alpha-activated endothelial cell monolayers, which express high levels of E-selectin, and CHO monolayers stably expressing E- or P-selectin. Further studies using CHO-E and -P monolayers demonstrate that the first 19 amino acids of PSGL-1 are sufficient for attachment and rolling on both E- and P-selectin and suggest that a sialyl Lewis x-containing glycan at Threonine-16 is critical for this sequence of amino acids to mediate attachment to E- and P-selectin. The data also demonstrate that a sulfated, anionic polypeptide segment within the amino terminus of PSGL-1 is necessary for PSGL-1-mediated attachment to P- but not to E-selectin. In addition, the results suggest that PSGL-1 has more than one binding site for E-selectin: one site located within the first 19 amino acids of PSGL-1 and one or more sites located between amino acids 19 through 148.
Figures









References
-
- Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature (Lond) 1995;374:539–542. - PubMed
-
- Arbones ML, Ord DC, Ley K, Radech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin (CD62L) deficient mice. Immunity. 1994;1:247–260. - PubMed
-
- Asa D, Raycroft L, Ma L, Aeed PA, Kaytes PS, Elhammer AP, Geng J. The P-selectin glycoprotein ligand functions as a common human leukocyte ligand for P- and E-selectins. J Biol Chem. 1995;270:11662–11670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

