Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse
- PMID: 9133368
- PMCID: PMC6573699
- DOI: 10.1523/JNEUROSCI.17-10-03425.1997
Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse
Abstract
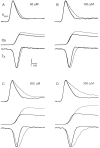
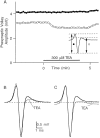
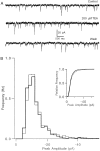
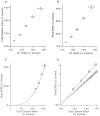
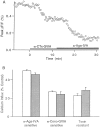
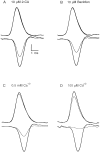
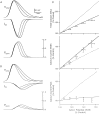
The effect of changes in the shape of the presynaptic action potential on neurotransmission was examined at synapses between granule and Purkinje cells in slices from the rat cerebellum. Low concentrations of tetraethylammonium were used to broaden the presynaptic action potential. The presynaptic waveform was monitored with voltage-sensitive dyes, the time course and amplitude of presynaptic calcium entry were determined with fluorescent calcium indicators, and EPSCs were measured with a whole-cell voltage clamp. Spike broadening increased calcium influx primarily by prolonging calcium entry without greatly affecting peak presynaptic calcium currents, indicating that the majority of calcium channels reach maximal probability of opening in response to a single action potential and that spike broadening increases the open time of these channels. EPSCs were exquisitely sensitive to elevations of calcium influx produced by spike broadening; there was a high power relationship between calcium influx and release such that a 23% increase in spike width led to a 25% increase in total calcium influx, which in turn doubled synaptic strength. The finding that even small changes in spike width influence neurotransmitter release suggests that altering the presynaptic waveform may be an important means of modifying the strength of this synapse. Waveform changes do not, however, contribute significantly to presynaptic modulation via activation of adenosine A1 or GABAB receptors. Furthermore, greatly reducing presynaptic calcium influx did not alter the presynaptic waveform, indicating that calcium channels and calcium-activated channels do not participate in shaping the presynaptic waveform.
Figures




Similar articles
-
Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels.J Neurosci. 2004 Jun 16;24(24):5623-31. doi: 10.1523/JNEUROSCI.0918-04.2004. J Neurosci. 2004. PMID: 15201335 Free PMC article.
-
Changes in action potential duration alter reliance of excitatory synaptic transmission on multiple types of Ca2+ channels in rat hippocampus.J Neurosci. 1996 Apr 1;16(7):2226-37. doi: 10.1523/JNEUROSCI.16-07-02226.1996. J Neurosci. 1996. PMID: 8601803 Free PMC article.
-
Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse.J Neurosci. 1996 Mar 1;16(5):1623-33. doi: 10.1523/JNEUROSCI.16-05-01623.1996. J Neurosci. 1996. PMID: 8774431 Free PMC article.
-
Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal network.Pflugers Arch. 2006 Dec;453(3):361-72. doi: 10.1007/s00424-006-0093-2. Epub 2006 Jun 27. Pflugers Arch. 2006. PMID: 16802161 Review.
-
Functions of Presynaptic Voltage-gated Calcium Channels.Function (Oxf). 2021;2(1):zqaa027. doi: 10.1093/function/zqaa027. Epub 2020 Oct 23. Function (Oxf). 2021. PMID: 33313507 Free PMC article. Review.
Cited by
-
Developmental transformation of the release modality at the calyx of Held synapse.J Neurosci. 2005 Apr 20;25(16):4131-40. doi: 10.1523/JNEUROSCI.0350-05.2005. J Neurosci. 2005. PMID: 15843616 Free PMC article.
-
Painful peripheral nerve injury decreases calcium current in axotomized sensory neurons.Anesthesiology. 2006 Jul;105(1):160-8. doi: 10.1097/00000542-200607000-00026. Anesthesiology. 2006. PMID: 16810008 Free PMC article.
-
Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity.J Neurosci. 2000 Dec 15;20(24):9162-73. doi: 10.1523/JNEUROSCI.20-24-09162.2000. J Neurosci. 2000. PMID: 11124994 Free PMC article.
-
Contribution of calcium-dependent facilitation to synaptic plasticity revealed by migraine mutations in the P/Q-type calcium channel.Proc Natl Acad Sci U S A. 2010 Oct 26;107(43):18694-9. doi: 10.1073/pnas.1009500107. Epub 2010 Oct 11. Proc Natl Acad Sci U S A. 2010. PMID: 20937883 Free PMC article.
-
Elementary mechanisms producing facilitation of Cav2.1 (P/Q-type) channels.J Gen Physiol. 2007 May;129(5):385-401. doi: 10.1085/jgp.200709749. Epub 2007 Apr 16. J Gen Physiol. 2007. PMID: 17438119 Free PMC article.
References
-
- Augustine GJ, Adler EM, Charlton MP. The calcium signal for transmitter secretion from presynaptic nerve terminals. In: Stanley EF, Nowycky MC, Triggle DJ, editors. Calcium entry and action at the presynaptic nerve terminal. New York Academy of Sciences; New York: 1991. pp. 365–381. - PubMed
-
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. - PubMed
-
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
